Abstract
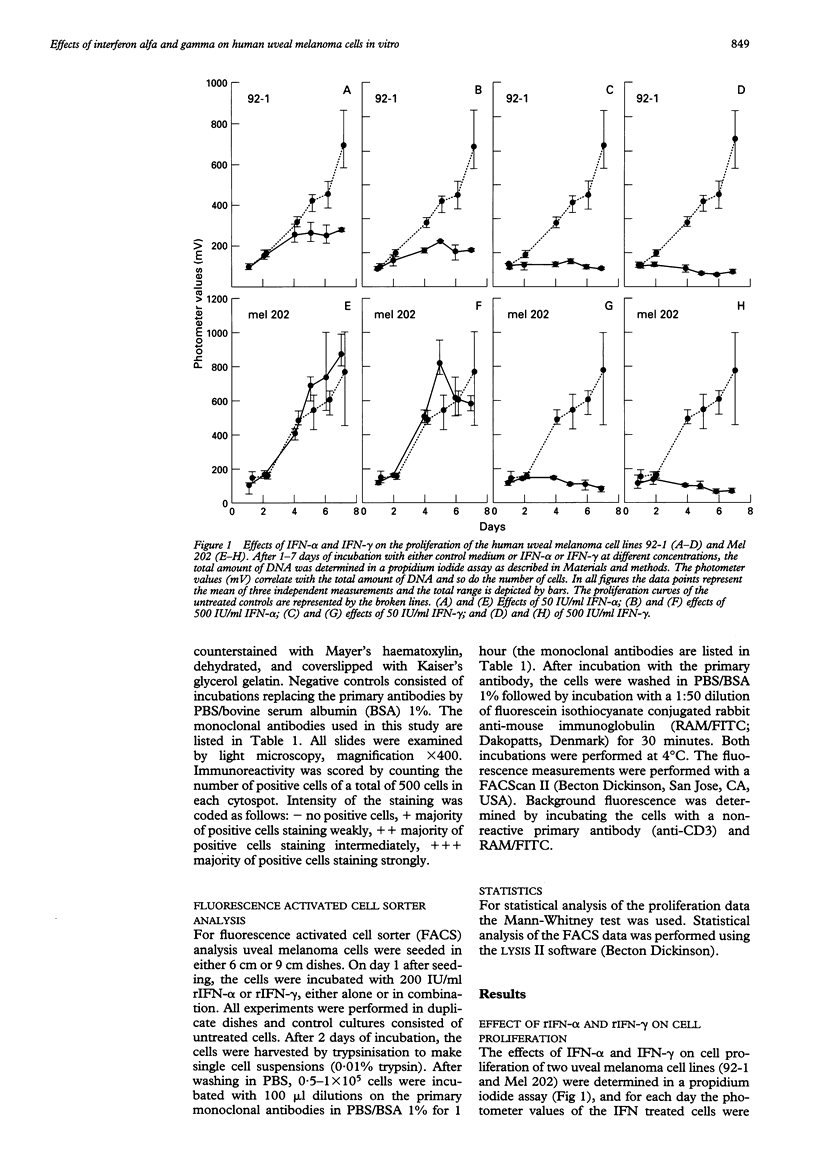
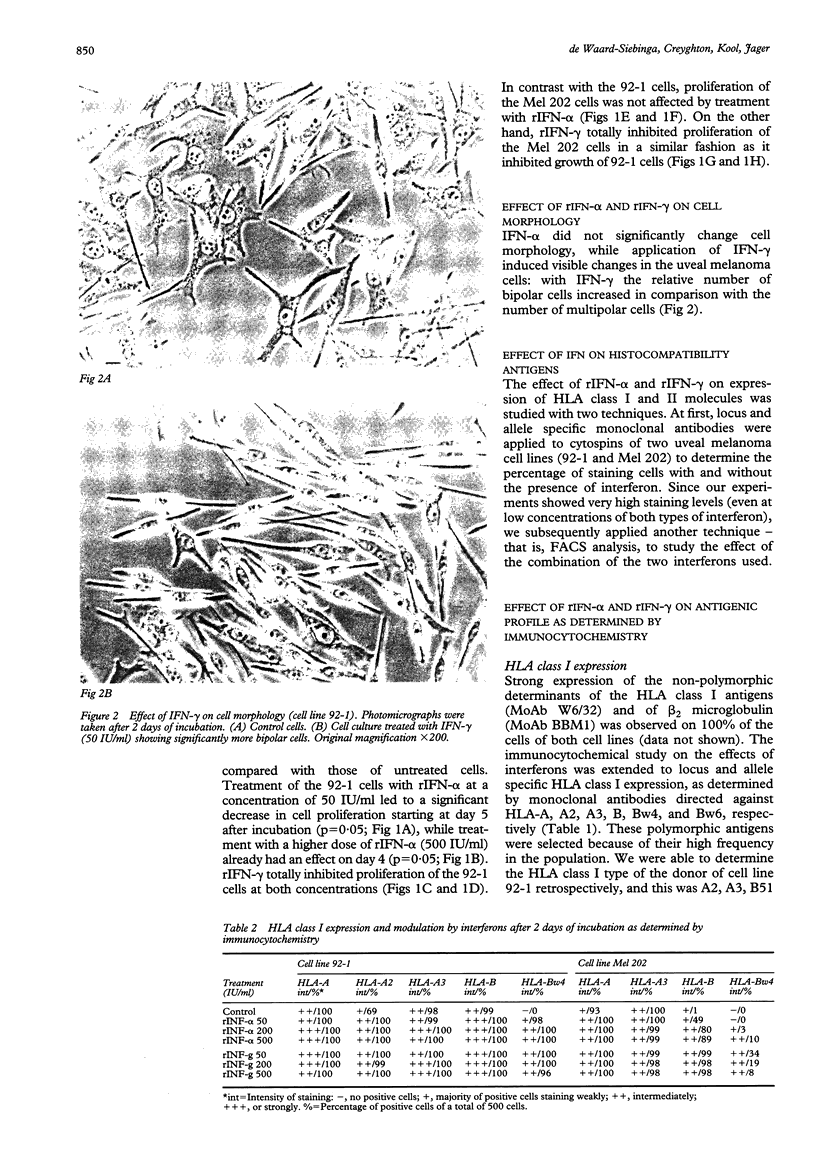
BACKGROUND--Uveal melanoma is a tumour with a high incidence of metastasis and a high mortality rate. Additional therapies to obtain a better local control or an effective treatment of metastases are necessary. Interferons may be applied. METHODS--The effects of human interferon alfa and gamma on proliferation and expression of immunologically important molecules of human uveal melanoma cells in vitro were studied. A propidium iodide assay was used to determine proliferation and immunostaining with monoclonal antibodies was applied to detect changes in antigen expression on two primary uveal melanoma cell lines, Mel 202 and 92-1. RESULTS--Interferon alfa inhibited proliferation of cell line 92-1 at a concentration of 50 IU/ml, but had no effect on cell line Mel 202, while interferon gamma inhibited growth of both cell lines. Only interferon gamma had a visible effect on cell morphology. With respect to the immunomodulatory effects, interferon alfa increased monomorphic HLA class I expression, but did not affect HLA class II expression. Interferon gamma induced not only HLA class I but also class II expression. The effects on HLA expression were locus-specific with the strongest effects observed for HLA-B and DR products. Small differences were observed with respect to the susceptibility of two different melanoma cell lines to antiproliferative effects and to modulation of antigen expression. CONCLUSION--The effects of interferon alfa and gamma on human uveal melanoma cells in vitro suggest a potential role of these cytokines in the treatment of patients with uveal melanoma. In particular, the immunomodulatory effects of these cytokines in vitro imply that treatment of patients with these cytokines might stimulate a beneficial antimelanoma immune response in vivo.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anichini A., Mortarini R., Fossati G., Parmiani G. Phenotypic profile of clones from early cultures of human metastatic melanomas and its modulation by recombinant interferon gamma. Int J Cancer. 1986 Oct 15;38(4):505–511. doi: 10.1002/ijc.2910380409. [DOI] [PubMed] [Google Scholar]
- Barnstable C. J., Bodmer W. F., Brown G., Galfre G., Milstein C., Williams A. F., Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978 May;14(1):9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Berger A. E., Davis J. E., Cresswell P. Monoclonal antibody to HLA-A3. Hybridoma. 1982;1(2):87–90. doi: 10.1089/hyb.1.1982.1.87. [DOI] [PubMed] [Google Scholar]
- Brodsky F. M., Bodmer W. F., Parham P. Characterization of a monoclonal anti-beta 2-microglobulin antibody and its use in the genetic and biochemical analysis of major histocompatibility antigens. Eur J Immunol. 1979 Jul;9(7):536–545. doi: 10.1002/eji.1830090709. [DOI] [PubMed] [Google Scholar]
- Carrel S., Schmidt-Kessen A., Giuffrè L. Recombinant interferon-gamma can induce the expression of HLA-DR and -DC on DR-negative melanoma cells and enhance the expression of HLA-ABC and tumor-associated antigens. Eur J Immunol. 1985 Feb;15(2):118–123. doi: 10.1002/eji.1830150204. [DOI] [PubMed] [Google Scholar]
- Clark R. H., Dimitrov N. V., Axelson J. A., Charamella L. J., Stott P. B. A phase II trial of intermittent leukocyte interferon and high dose chlorambucil in the treatment of non-Hodgkin's lymphoma resistant to conventional therapy. Am J Clin Oncol. 1989 Feb;12(1):75–77. doi: 10.1097/00000421-198902000-00017. [DOI] [PubMed] [Google Scholar]
- Clemens M. J., McNurlan M. A. Regulation of cell proliferation and differentiation by interferons. Biochem J. 1985 Mar 1;226(2):345–360. doi: 10.1042/bj2260345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley N. J., Darrow T. L., Quinn-Allen M. A., Seigler H. F. MHC-restricted recognition of autologous melanoma by tumor-specific cytotoxic T cells. Evidence for restriction by a dominant HLA-A allele. J Immunol. 1991 Mar 1;146(5):1692–1699. [PubMed] [Google Scholar]
- De Waard-Siebinga I., Blom D. J., Griffioen M., Schrier P. I., Hoogendoorn E., Beverstock G., Danen E. H., Jager M. J. Establishment and characterization of an uveal-melanoma cell line. Int J Cancer. 1995 Jul 17;62(2):155–161. doi: 10.1002/ijc.2910620208. [DOI] [PubMed] [Google Scholar]
- Detrick B., Evans C. H., Chader G., Percopo C. M., Hooks J. J. Cytokine-induced modulation of cellular proteins in retinoblastoma. Analysis by flow cytometry. Invest Ophthalmol Vis Sci. 1991 May;32(6):1714–1722. [PubMed] [Google Scholar]
- Fisher P. B., Grant S. Effects of interferon on differentiation of normal and tumor cells. Pharmacol Ther. 1985;27(2):143–166. doi: 10.1016/0163-7258(85)90067-1. [DOI] [PubMed] [Google Scholar]
- Giacomini P., Gambari R., Barbieri R., Nisticò P., Tecce R., Pestka S., Gustafsson K., Natali P. G., Fisher P. B. Regulation of the antigenic phenotype of human melanoma cells by recombinant interferons. Anticancer Res. 1986 Sep-Oct;6(5):877–884. [PubMed] [Google Scholar]
- Giacomini P., Tecce R., Gambari R., Sacchi A., Fisher P. B., Natali P. G. Recombinant human IFN-gamma, but not IFN-alpha or IFN-beta, enhances MHC- and non-MHC-encoded glycoproteins by a protein synthesis-dependent mechanism. J Immunol. 1988 May 1;140(9):3073–3081. [PubMed] [Google Scholar]
- Goldberg R. M., Ayoob M., Silgals R., Ahlgren J. D., Neefe J. R. Phase II trial of lymphoblastoid interferon in metastatic malignant melanoma. Cancer Treat Rep. 1985 Jul-Aug;69(7-8):813–816. [PubMed] [Google Scholar]
- Goldstein D., Laszlo J. Interferon therapy in cancer: from imaginon to interferon. Cancer Res. 1986 Sep;46(9):4315–4329. [PubMed] [Google Scholar]
- Golomb H. M., Fefer A., Golde D. W., Ozer H., Portlock C., Silber R., Rappeport J., Ratain M. J., Thompson J., Bonnem E. Report of a multi-institutional study of 193 patients with hairy cell leukemia treated with interferon-alfa2b. Semin Oncol. 1988 Oct;15(5 Suppl 5):7–9. [PubMed] [Google Scholar]
- Ishii Y., Tsukagoshi S. The synergistic effect of human recombinant interferon-alpha 2a in combination with interferon-gamma and the induction of interferon-alpha 2a receptor by interferon-gamma. J Pharmacobiodyn. 1989 May;12(5):299–304. doi: 10.1248/bpb1978.12.299. [DOI] [PubMed] [Google Scholar]
- Ksander B. R., Rubsamen P. E., Olsen K. R., Cousins S. W., Streilein J. W. Studies of tumor-infiltrating lymphocytes from a human choroidal melanoma. Invest Ophthalmol Vis Sci. 1991 Dec;32(13):3198–3208. [PubMed] [Google Scholar]
- Ledbetter J. A., Evans R. L., Lipinski M., Cunningham-Rundles C., Good R. A., Herzenberg L. A. Evolutionary conservation of surface molecules that distinguish T lymphocyte helper/inducer and cytotoxic/suppressor subpopulations in mouse and man. J Exp Med. 1981 Feb 1;153(2):310–323. doi: 10.1084/jem.153.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maio M., Gulwani B., Langer J. A., Kerbel R. S., Duigou G. J., Fisher P. B., Ferrone S. Modulation by interferons of HLA antigen, high-molecular-weight melanoma associated antigen, and intercellular adhesion molecule 1 expression by cultured melanoma cells with different metastatic potential. Cancer Res. 1989 Jun 1;49(11):2980–2987. [PubMed] [Google Scholar]
- McLeod G. R., Thomson D. B., Hersey P. Clinical evaluation of interferons in malignant melanoma. J Invest Dermatol. 1990 Dec;95(6 Suppl):185S–187S. doi: 10.1111/1523-1747.ep12875500. [DOI] [PubMed] [Google Scholar]
- Munker M., Munker R., Saxton R. E., Koeffler H. P. Effect of recombinant monokines, lymphokines, and other agents on clonal proliferation of human lung cancer cell lines. Cancer Res. 1987 Aug 1;47(15):4081–4085. [PubMed] [Google Scholar]
- Murray J. L., Rosenblum M. G. Modulation of melanoma antigens by interferons. Cancer Treat Res. 1991;54:153–167. doi: 10.1007/978-1-4615-3938-4_9. [DOI] [PubMed] [Google Scholar]
- Nisticò P., Tecce R., Giacomini P., Cavallari A., D'Agnano I., Fisher P. B., Natali P. G. Effect of recombinant human leukocyte, fibroblast, and immune interferons on expression of class I and II major histocompatibility complex and invariant chain in early passage human melanoma cells. Cancer Res. 1990 Dec 1;50(23):7422–7429. [PubMed] [Google Scholar]
- Osanto S., Jansen R., Naipal A. M., Gratama J. W., van Leeuwen A., Cleton F. J. In vivo effects of combination treatment with recombinant interferon-gamma and -alpha in metastatic melanoma. Int J Cancer. 1989 Jun 15;43(6):1001–1006. doi: 10.1002/ijc.2910430608. [DOI] [PubMed] [Google Scholar]
- Osanto S., Jansen R., Vloemans M. Downmodulation of c-myc expression by interferon gamma and tumour necrosis factor alpha precedes growth arrest in human melanoma cells. Eur J Cancer. 1992;28A(10):1622–1627. doi: 10.1016/0959-8049(92)90055-7. [DOI] [PubMed] [Google Scholar]
- Parham P., Brodsky F. M. Partial purification and some properties of BB7.2. A cytotoxic monoclonal antibody with specificity for HLA-A2 and a variant of HLA-A28. Hum Immunol. 1981 Dec;3(4):277–299. doi: 10.1016/0198-8859(81)90065-3. [DOI] [PubMed] [Google Scholar]
- Radka S. F., Kostyu D. D., Amos D. B. A monoclonal antibody directed against the HLA-Bw6 epitope. J Immunol. 1982 Jun;128(6):2804–2806. [PubMed] [Google Scholar]
- Rebai N., Malissen B., Pierres M., Accolla R. S., Corte G., Mawas C. Distinct HLA-DR epitopes and distinct families of HLA-Dr molecules defined by 15 monoclonal antibodies (mAb) either anti-DR or allo-anti-Iak cross-reacting with human DR molecule. I. Cross-inhibition studies of mAb cell surface fixation and differential binding of mAb to detergent-solubilized HLA molecules immobilized to a solid phase by a first mAb. Eur J Immunol. 1983 Feb;13(2):106–111. doi: 10.1002/eji.1830130205. [DOI] [PubMed] [Google Scholar]
- Schanz U., Roelen D. L., Bruning J. W., Kardol M. J., van Rood J. J., Claas F. H. The relative radioresistance of interleukin-2 production by human peripheral blood lymphocytes: consequences for the development of a new limiting dilution assay for the enumeration of helper T lymphocyte precursor frequencies. J Immunol Methods. 1994 Mar 10;169(2):221–230. doi: 10.1016/0022-1759(94)90266-6. [DOI] [PubMed] [Google Scholar]
- Spits H., Borst J., Giphart M., Coligan J., Terhorst C., De Vries J. E. HLA-DC antigens can serve as recognition elements for human cytotoxic T lymphocytes. Eur J Immunol. 1984 Apr;14(4):299–304. doi: 10.1002/eji.1830140404. [DOI] [PubMed] [Google Scholar]
- Stam N. J., Spits H., Ploegh H. L. Monoclonal antibodies raised against denatured HLA-B locus heavy chains permit biochemical characterization of certain HLA-C locus products. J Immunol. 1986 Oct 1;137(7):2299–2306. [PubMed] [Google Scholar]
- Stam N. J., Vroom T. M., Peters P. J., Pastoors E. B., Ploegh H. L. HLA-A- and HLA-B-specific monoclonal antibodies reactive with free heavy chains in western blots, in formalin-fixed, paraffin-embedded tissue sections and in cryo-immuno-electron microscopy. Int Immunol. 1990;2(2):113–125. doi: 10.1093/intimm/2.2.113. [DOI] [PubMed] [Google Scholar]
- Talpaz M., Kantarjian H. M., McCredie K., Trujillo J. M., Keating M. J., Gutterman J. U. Hematologic remission and cytogenetic improvement induced by recombinant human interferon alpha A in chronic myelogenous leukemia. N Engl J Med. 1986 Apr 24;314(17):1065–1069. doi: 10.1056/NEJM198604243141701. [DOI] [PubMed] [Google Scholar]
- Taramelli D., Fossati G., Mazzocchi A., Delia D., Ferrone S., Parmiani G. Classes I and II HLA and melanoma-associated antigen expression and modulation on melanoma cells isolated from primary and metastatic lesions. Cancer Res. 1986 Jan;46(1):433–439. [PubMed] [Google Scholar]
- Traversari C., van der Bruggen P., Luescher I. F., Lurquin C., Chomez P., Van Pel A., De Plaen E., Amar-Costesec A., Boon T. A nonapeptide encoded by human gene MAGE-1 is recognized on HLA-A1 by cytolytic T lymphocytes directed against tumor antigen MZ2-E. J Exp Med. 1992 Nov 1;176(5):1453–1457. doi: 10.1084/jem.176.5.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyring S. K., Klimpel G., Brysk M., Gupta V., Stanton G. J., Fleischmann W. R., Jr, Baron S. Eradication of cultured human melanoma cells by immune interferon and leukocytes. J Natl Cancer Inst. 1984 Nov;73(5):1067–1073. [PubMed] [Google Scholar]
- Watson A. J., DeMars R., Trowbridge I. S., Bach F. H. Detection of a novel human class II HLA antigen. 1983 Jul 28-Aug 3Nature. 304(5924):358–361. doi: 10.1038/304358a0. [DOI] [PubMed] [Google Scholar]
- Wölfel T., Klehmann E., Müller C., Schütt K. H., Meyer zum Büschenfelde K. H., Knuth A. Lysis of human melanoma cells by autologous cytolytic T cell clones. Identification of human histocompatibility leukocyte antigen A2 as a restriction element for three different antigens. J Exp Med. 1989 Sep 1;170(3):797–810. doi: 10.1084/jem.170.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]