Figure 3.
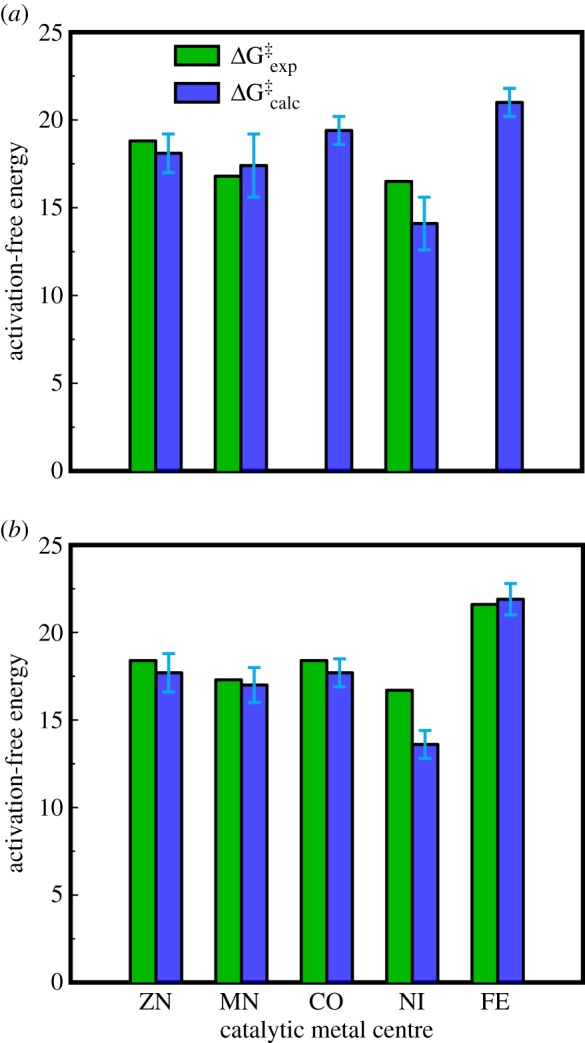
Comparison of the experimental (ΔG‡exp, green) and calculated (ΔG‡calc, blue) activation-free energies for the hydrolysis of (a) paraoxon and (b) p-nitrophenyl butyrate by MPH in complex with different metal ions. Error bars on the calculated values represent standard deviations calculated over 10 discrete EVB trajectories for each substrate and metal ion. The corresponding data are shown in electronic supplementary material, tables S1 and S2, and ΔG‡exp was calculated from kinetic data presented in [17]. Note that in the two cases where experimental data is not presented in panel (a), it was not possible to obtain kcat [17].
