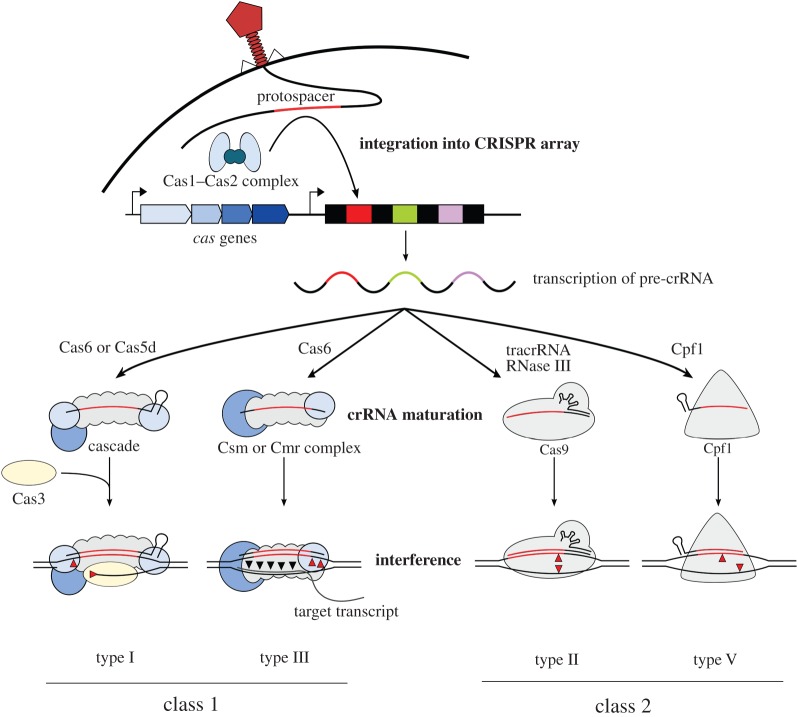
Figure 1.
Simplified model of the immunity mechanisms of class 1 and class 2 CRISPR-Cas systems. The CRISPR-Cas systems are composed of a cas operon (blue arrows) and a CRISPR array that comprises identical repeat sequences (black rectangles) that are interspersed by phage-derived spacers (coloured rectangles). Upon phage infection, a sequence of the invading DNA (protospacer) is incorporated into the CRISPR array by the Cas1–Cas2 complex. The CRISPR array is then transcribed into a long precursor CRISPR RNA (pre-crRNA), which is further processed by Cas6 in type I and III systems (processing in type I-C CRISPR-Cas systems by Cas5d). In type II CRISPR-Cas systems, crRNA maturation requires tracrRNA, RNase III and Cas9, whereas in type V-A systems Cpf1 alone is sufficient for crRNA maturation. In the interference state of type I systems, Cascade is guided by crRNA to bind the foreign DNA in a sequence-specific manner and subsequently recruits Cas3 that degrades the displaced strand through its 3′–5′ exonucleolytic activity. Type III-A and type III-B CRISPR-Cas systems employ Csm and Cmr complexes, respectively, for cleavage of DNA (red triangles) and its transcripts (black triangles). A ribonucleoprotein complex consisting of Cas9 and a tracrRNA : crRNA duplex targets and cleaves invading DNA in type II CRISPR-Cas systems. The crRNA-guided effector protein Cpf1 is responsible for target degradation in type V systems. Red triangles represent the cleavage sites of the interference machinery.