Abstract
Background
Approximately half of patients with chronic cardiometabolic conditions are non-adherent with their prescribed medications. Interventions to improve adherence have been only modestly effective because they often address single barriers to adherence, intervene at single points in time, or are imprecisely targeted to patients who may or may not need adherence assistance.
Objective
To evaluate the effect of a multi-component, behaviorally-tailored pharmacist-based intervention to improve adherence to medications for diabetes, hypertension, and hyperlipidemia.
Trial design
The STIC2IT (Study of a Tele-pharmacy Intervention for Chronic diseases To Improve Treatment adherence) trial is a cluster-randomized pragmatic trial testing the impact of a pharmacist-led multi-component intervention that uses behavioral interviewing, text messaging, mailed progress reports and video visits. Targeted patients are those who are non-adherent to glucose-lowering, anti-hypertensive, or statin medications and who also have evidence of poor disease control. The intervention is tailored to patients’ individual health barriers and their level of health activation. We cluster randomized 14 practice sites of a large multi-specialty group practice to receive either the pharmacist-based intervention or usual care. STIC2IT has enrolled 4,076 patients to be followed for 12 months after randomization. The trial’s primary outcome is medication adherence, assessed using pharmacy claims data. Secondary outcomes are disease control and healthcare resource utilization.
Conclusion
This trial will determine whether a technologically-enabled, behaviorally-targeted pharmacist-based intervention results in improved adherence and disease control. If effective, this strategy could be a scalable method of offering tailored adherence support to those with the greatest clinical need.
BACKGROUND
While rates of prescribing evidence-based therapies for cardiovascular and other chronic conditions have improved substantially, long-term adherence remains poor.1,2,3 Nearly one-half of patients become non-adherent within a year of treatment initiation1,2,3,4 with adverse consequences on morbidity and mortality.4 The avoidable healthcare costs attributable to medication non-adherence have been estimated to be anywhere from $100 to $300 billion in the US annually, representing 3% to 10% of total US health care spending.5,6
Barriers to medication adherence arise from a complex interplay among patient, provider, and system-related factors.7,8 As a result, strategies to improve adherence vary widely including educational interventions with behavioral support, case management, reminder calls, telephone-based counseling, decision aides, text messages, electronic pills bottles and policy interventions that reduce out-of-pocket expenses for prescription medications.9,10,11 Unfortunately, when rigorously tested, many of these approaches have only been moderately successful.9,12,13,14,15 This limited efficacy may reflect that many interventions address a single barrier to adherence, do so at a single point in time, and are imprecisely targeted with respect to which patients are most likely to benefit and how the intervention is tailored to meet their specific needs.16 Among those interventions demonstrating success, many have not been widely adopted because of the substantial resources required to deliver and sustain them.17
To address these limitations, we launched the Study of a Tele-pharmacy Intervention for Chronic diseases to (2) Improve Treatment adherence (STIC2IT) trial.
OVERALL STUDY DESIGN
STIC2IT is a pragmatic, prospective, intention-to-treat, cluster-randomized controlled trial designed to test the impact of a technologically-enabled, behaviorally-targeted pharmacist intervention designed to improve medication adherence and disease control among the specific group of individuals who are most likely to benefit from this intervention – those who are non-adherent to their glucose lowering, anti-hypertensive, or statin medications and have evidence of poor disease control based on recommended clinical targets. The trial is funded by the U.S. National Institutes of Health National Heart, Lung, and Blood Institute was approved by the institutional review board of Brigham and Women’s Hospital, and is registered with clinicaltrials.gov (NCT02512276). The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
STUDY SETTING AND RANDOMIZATION
This trial is being conducted at Harvard Vanguard Medical Associates (Harvard Vanguard), which is a practice of Atrius Health, a large multi-specialty medical group and a Pioneer Accountable Care Organization. Harvard Vanguard employs approximately 150 primary care physicians (PCPs) who provide care for approximately 300,000 adult patients at 17 practice sites. Of these, 15 practice sites have integrated retail pharmacies, where approximately 50% of patients obtain their prescription medications.
We randomly selected one of the 15 Harvard Vanguard practice sites with onsite pharmacies as a pilot site for intervention refinement. The remaining 14 Harvard Vanguard practice sites were then cluster-randomized such that all PCPs and their patients in a given practice site were assigned to the same study arm. Because the practice sites differ from each other, simple cluster randomization may have resulted in imbalances in patient or provider factors that could potentially bias outcome assessment. Therefore, we categorized the practice sites based on their size (i.e., small or large, based on the number of patients receiving care at each site) and whether clinical pharmacists at the sites offered disease management counseling directly to patients (i.e., yes or no). Within the resultant 4 blocks, practices were then randomized in a 1:1 ratio to intervention or control using a random number generator.
SUBJECTS
Study enrollment began in August 2015 and was completed in July 2016. Follow-up of all trial participants will end in July 2017. In total, 4076 patients have been randomized. Potentially eligible patients for inclusion are those who: (1) are receiving care from a Harvard Vanguard PCP and who are also receiving health insurance from one of 4 large health insurers, (2) have evidence of poor or worsening disease control (Table 1), and (3) are identified as non-adherent (as defined below) to their oral glucose-lowering, anti-hypertensive, or statin medications.
TABLE 1.
Definitions of poor and worsening disease control
| Condition | Age (in years) | Poor control | Worsening control |
|---|---|---|---|
| Diabetes18 | … | Latest HbA1c > 8 | Latest HbA1C 7.5 ≤ to ≤ 8, and previous HbA1C 1% lower |
| Hypertension19 | ≥ 60 | Latest BP > 150/90 | Latest BP 140/80 ≤ to ≤ 150/90, and previous BP 20 mmHg lower |
| < 60 | Latest BP > 140/90 | Latest BP 130/80 ≤ to ≤ 140/90, and previous systolic or diastolic BP 20 mmHg lower | |
| Hyperlipidemia20 | … | Diagnoses of ASCVD | … |
| 40–75 | Type 1 or 2 diabetes and use of glucose lowering agent | … | |
| 40–79 | ASCVD risk > 7.5% | … | |
| … | LDL>190 mg/dl | Latest LDL 175 ≤ to ≤ 190, and previous LDL 30 mg/dl lower |
…= N/A; Abbreviations: HbA1c, glycosylated hemoglobin; BP, blood pressure; ASCVD, atherosclerotic cardiovascular disease; LDL, low-density lipoprotein
Disease control is evaluated using the most recent lab or blood pressure values in the electronic health record and is based on clinical guideline targets from the American Diabetes Association (ADA), the Eighth Joint National Committee (JNC 8) hypertension guidelines, and the American College of Cardiology/American Heart Association (ACC/AHA) cholesterol guidelines.18,19,20 Adherence is assessed using prescription claims data. For each medication used to treat any one of the targeted conditions, the proportion of days covered (PDC) is calculated as the number of days of medication that a patient filled between the first fill date and the randomization date divided by the number of days in that same period (up to a maximum of 365 days).2 We consider drugs that are chemically related and not intended for use in combination to be interchangeable (e.g., two different statins). Using previously described methods,21 we average the PDC for all of the medications used to treat a single condition (e.g., all oral hypoglycemics) and then calculate an overall average adherence for all of the conditions that a patient had at the time of their identification. In order to identify individuals who would benefit most from the intervention, a patient is defined as being non-adherent if they (1) have been less than 80% adherent to the specific class of medications used to treat the condition(s) for which they were being identified as being poorly controlled and (2) if their “average of averages” PDC for all eligible study drugs is less than 80%. For example, patients with poorly controlled diabetes who are non-adherent to their diabetes medications but adherent to their statins and antihypertensives, would only be eligible if their average adherence across all 3 conditions is less than 80%. Patients are excluded if, prior to randomization, they have less than 6 months of continuous enrollment in the health plan (to allow adequate assessment of eligibility), are < 18 or > 85 years of age, or have no available telephone contact information, which would preclude contact for enrollment and delivery of the intervention.
STUDY PROCEDURES
Once identified, PCPs of potentially eligible intervention group patients are sent a secure message using the electronic health record to ask permission to include their patient(s) in the study. Based on prior agreement with the practices, if a physician does not explicitly respond to approve or disapprove of the identified patient(s) to participate in the study within 5 days, they are sent a reminder message; one day later, their patients are automatically opted in to the study.
Patients approved for enrollment in the study are sent a letter informing them about the study along with a simple, one compartment per day, pillbox that allows for the storage of one week of medication. Patients are then contacted by telephone to schedule a phone consultation with the clinical pharmacist. At this time, they are also administered the Patient Activation Measure (PAM), a questionnaire to assess the knowledge, skills, and confidence to manage one’s health and health care.22,23 As described below, the PAM is subsequently used to tailor the intervention for that individual patient.
INTERVENTION
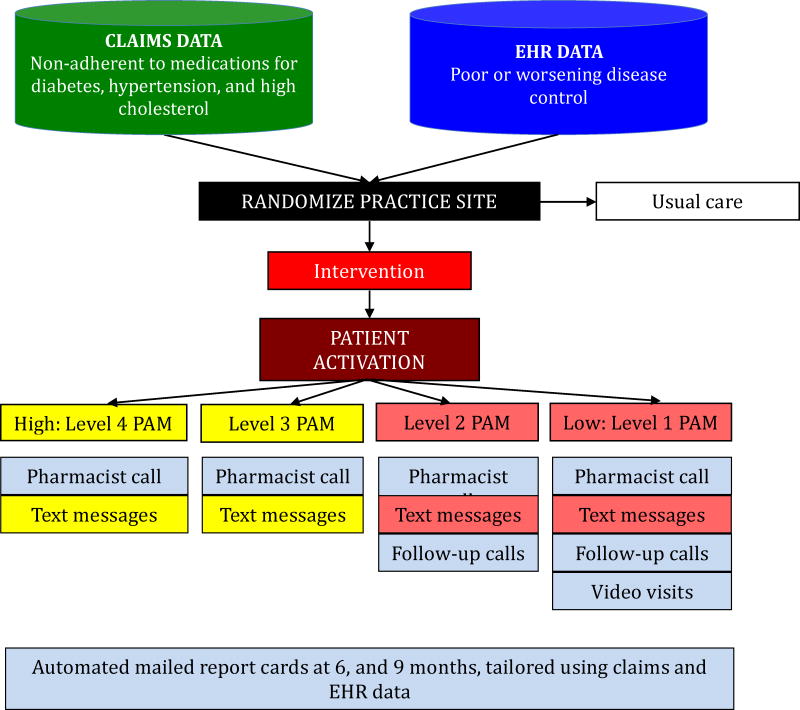
The central component of the multi-faceted intervention is an individually-tailored telephone consultation conducted by a clinical pharmacist who is part of the Harvard Vanguard care team (Figure 1). The clinical pharmacists work at multiple sites; in order to reduce the chances of contamination, they are restricted to providing clinical services only at other intervention sites during the time period of this study.
FIGURE 1.
Study design and intervention components
During this consultation, the clinical pharmacist confirms a patient’s treatment regimen, engages the patient in sharing potential barriers to adherence or other factors that may be contributing to poor disease control, discusses the patient’s readiness to modify behaviors, and works with the patient to agree upon a shared plan of strategies to improve adherence and disease control (see Appendix A). The identified adherence barriers are grouped into six distinct groups: treatment complexity/forgetfulness, health perceptions, lack of knowledge/poor health literacy, side effects, cognitive impairment, and cost-related barriers. Patients may have barriers identified in more than one category. The solutions and strategies offered to patients by the clinical pharmacists are tailored to their PAM level and their identified adherence barrier(s) (see Table 2). Depending on the barrier, patients with lower levels of activation (e.g., PAM Level 1 and 2) are offered more intensive solutions, such as daily text messages as reminders or motivational support, pillboxes that allow for multiple times per day dosing, follow-up consultations, and video visits through the WebEx platform (Santa Clara, CA). The video visits allow for one-on-one communication, delivered remotely. Patients with higher levels of activation (e.g., PAM Level 3 and 4) are offered less intensive solutions, such as weekly text messages and pillboxes.23 The clinical pharmacists then work with the primary care physicians and other team members at Atrius Health to implement solutions based on the treatment plan. The clinical pharmacists also mail a copy of the shared plan to the patients after the initial encounter.
TABLE 2.
Patient barriers to adherence and available solutions
| BARRIER | SOLUTION | SPECIFIC STRATEGY | |
|---|---|---|---|
| LOW ACTIVATION (PAM 1 and PAM 2) | HIGH ACTIVATION (PAM 3 and PAM 4) | ||
| Treatment complexity and/or forgetfulness | Medication review |
|
|
| Pill organization and reminders |
|
|
|
| Counseling |
|
|
|
| Text messaging |
|
|
|
| Family and/or social work involvement |
|
|
|
| Health perception | Counseling |
|
|
| Text messaging |
|
|
|
| Cognitive impairment | Family and/or social work involvement |
|
|
| Counseling, education |
|
|
|
| Lack of knowledge and/or poor health literacy | Counseling |
|
|
| Family and/or social work involvement |
|
|
|
| Experiencing side effects | Medication review |
|
|
| Counseling |
|
|
|
| Costs | Medication review |
|
|
| Mail order, social work support |
|
|
|
| Counseling |
|
|
|
| All barriers | Follow-up calls |
|
|
PAM = Patient Activation Measure; PCP = Primary Care Physician
The structure of the initial and follow-up clinical pharmacists phone calls was developed by the study team (see Appendix A and B), based on the principles of brief negotiated interview (BNI),24,25,26 a behavioral interviewing technique with foundations in motivational interviewing, and refined during the pilot phase of the trial. Prior to the start of the study, the clinical pharmacists underwent a full-day training program that included script development and role-playing exercises with feedback by the study team. These role-playing exercises were then repeated two more times. The initial calls last 30 to 45 minutes. Follow-up calls are scheduled with all patients with low levels of activation but only if clinically indicated for highly activated individuals.
Depending on the barrier(s) identified, patients are offered the opportunity to receive SMS text messages via a secure messaging platform (Mobile Commons; Brooklyn, NY) for the 12-month follow-up period or until the patient opts out. These 50 unique text messages were developed by the study team to provide reminders and motivation to subjects who opted to receive them (see Appendix C). In addition to the motivational text messages, patients are asked questions about their adherence behavior to which they can provide answers by directly replying to the text message and receive automated responses using a feedback response system. The response system provides different encouraging feedback or advice, depending on the patients’ inputs. The content and frequency of the text messages differ depending on patients’ PAM levels and adherence barrier. For example, patients with lower levels of activation are more frequently asked specific questions about their adherence behavior, are encouraged to reply more often, and are also offered the choice of receiving daily or weekly texts, whereas patients with higher levels of activation are only offered weekly texts.
All intervention patients who do not opt out of trial participation are mailed progress reports at 6 months, and 9 months after randomization on behalf of their PCP. These progress reports provide personalized and updated information about disease control generated using data from the electronic health record. For patients whose administrative claims data are believed by the clinical pharmacist to be an accurate representation of actual filling behavior (e.g., for patients who only use their prescription insurance plan to pay for their prescriptions), the progress reports also provide patient-specific medication adherence information.
The specific components of the intervention that are administered to each patient, including the number, frequency and length of the phone consultations that they receive, are being explicitly tracked to facilitate future reproducibility and scalability.
OUTCOMES
The trial’s primary outcome is medication adherence assessed at 12 months after randomization (see Table 3). Medication adherence will be assessed using prescription claims data and measured as the mean PDC over the 12 months after randomization using the “average of averages” approach used for study eligibility. Adherence will be measured only for medications that qualified a patient for inclusion in the study and follow-up will begin at the point of randomization.21 Medications that were filled prior to randomization but had a supply that extended into the follow-up period will have their carry-over supply included in the adherence calculation. In a sensitivity analysis of this outcome, medication adherence will be measured by calculating PDC beginning from the first fill of a medication after randomization until the end of the 12-month follow-up period. As a second sensitivity analysis, we will also censor patients when they initiate insulin.
TABLE 3.
Study outcomes and their measurements
| Type | Outcome | Definition* |
|---|---|---|
| Primary | Medication adherence | Average proportion of days covered for medications to treat eligible conditions |
| Secondary | Disease control | Proportion of patients achieving good disease control for all eligible conditions |
| Proportion of patients achieving good disease control for at least one eligible condition | ||
| Healthcare utilization | Rates of all-cause emergency room visits, physician office visits, and hospitalizations |
Please see text for additional details
The secondary outcomes for the study include disease control and rates of healthcare utilization. Disease control will be measured as the following two different outcomes: (1) the proportion of patients achieving good disease control for all of their eligible conditions and (2) the proportion of patients achieving good disease control for at least one of their eligible conditions. Because disease control will be evaluated using biometrics that are collected during routine care rather than at study-prescribed intervals, we will use those values that are closest to each patient’s 12-month end of follow-up period.
Rates of healthcare utilization will also be measured using administrative claims data and will include all-cause emergency room visits, physician office visits, and hospitalizations during follow-up. In addition, patients in the intervention group will be administered a mailed survey 12 months after randomization to assess self-reported adherence. Self-reported adherence will be assessed as the proportion of patients who are deemed adherent according to the a validated 3-item self-report measure for medication adherence.27 Patients will be contacted by telephone if they do not respond to the mailed survey.
ANALYTIC PLAN
We will report the means and frequencies of pre-randomization variables separately for intervention and control subjects. Comparisons of these values will be performed using t-tests and chi square tests and their non-parametric analogs, as appropriate. The outcomes will be evaluated using intention-to-treat principles among all randomized patients. In the primary analysis, the outcomes will be compared using generalized estimating equations with an identity link function and normally distributed errors to account for the clustering of subjects within practice sites. Our primary models will also adjust for the block-randomized design. If there are differences in baseline characteristics between study groups that are believed to be confounders of the intervention–outcome association, we will repeat our analyses after adjusting for these covariates. A similar approach will be taken for the analysis of the secondary outcomes, except using logit link functions with binary errors and log link functions with Poisson errors, as appropriate. If more than 10% of subjects have missing outcome data, we will repeat our analyses using the latest post-randomization lab values available and using multiple imputation.28
Several additional analyses will also be conducted. First, we will assess the correlation between calculated adherence based on insurer claims, self-reported adherence, and pharmacy transaction records (for patients filling prescriptions at Harvard Vanguard pharmacies). Second, we will use Markov modeling to assess the long-term impact of the intervention on cost, quality-adjusted survival, and cost-effectiveness of the intervention.
SAMPLE SIZE CONSIDERATIONS
Our study should be sufficiently powered to detect small, clinically meaningful changes in the primary outcome. We powered the study to detect a 2.5% mean change in adherence between the intervention and control groups, assuming a standard deviation of 0.25 (a conservative assumption), clustering at the practice level with a design effect of 1.10,29,30 and a 15% non-differential loss to follow-up rate. We assumed that 95% of potentially eligible intervention patients would be approved for study inclusion by their PCPs and that 50% of these patients would agree to a pharmacist consultation. With these assumptions, we estimated that we would need a total sample size of 4000 eligible patients to provide more than 80% power to detect differences in our primary study outcome of medication adherence. In other words, if those patients receiving the intervention demonstrate a 4.6% improvement in adherence, we will still have more than 80% power to detect an improvement of 2.5% in the overall sample. We also have substantial power to detect improvements in disease control, assuming a rate of controlled disease of 30% based on our pilot data.
To reach our enrollment target while ensuring that all identified patients can be contacted and intervened upon in a timely fashion, we are querying the electronic health records and claims data every two weeks and selecting a random sample of 85 patients from all eligible patients for each of the intervention and control groups. After identification, selected patients are removed from the pool of potentially eligible patients in subsequent rounds. Each patient’s randomization date is defined as the day of the biweekly data query on which they are identified as being eligible for inclusion in the study.
LIMITATIONS
There are several limitations to this trial that should be acknowledged. First, patients who fill their prescriptions by paying cash or using low-cost generic programs will not have adjudicated claims reflecting these transactions and thus may be misclassified as being non-adherent.31,32 While we are prospectively tracking intervention patients who are identified as having missing or inaccurate claims because of cash payments, we expect any misclassification introduced by this claims inaccuracy to be non-differential between the study arms. Further, our ability to assess the impact of the intervention on disease control is unaffected this claims inaccuracy. Second, claims-based methods of adherence estimation may be prone to misclassification as they cannot differentiate between patients who discontinue or switch medications based on their doctors’ orders as opposed to not filling their medication due to non-adherence. We will assess the robustness of our results using alternate definitions of the primary outcome measure of adherence, as described above. In addition, our primary outcome definition is more conservative by design, because clinical pharmacist-directed switching may be more likely to occur in the intervention group. Further, claims-based methods of adherence assessment have been shown to correlate highly with patient self-report, pill counts and serum drug levels33,34 and are also the basis for quality metrics currently being used by Medicare and other agencies.35 Third, patients may not have a lab value or blood pressure reading in the 12 month follow-up period, particularly given the newer cholesterol-lowering guidelines, which may lead to incomplete assessment of clinical outcomes. Fourth, while multi-component adherence interventions have consistently been found to be more effective than those addressing single barriers, should the trial meet its primary outcome, we will not be able to identify which aspect of the intervention was responsible for the effect. Finally, while our trial is pragmatic in its design and leverages, to the extent possible, interventions that are relatively low cost, there is substantial infrastructure necessary to administer the intervention. As such, our results may not be generalizable to all existing care settings or patients, such as those without reliable access to a telephone or the other technologies we are employing. However, because telephone and cellphone access is almost universal in the U.S., our results should apply to the vast majority of patients being treated in the rapidly expanding number of accountable care organizations, patient centered medical homes and other similar integrated care models.
CONCLUSION
This cluster-randomized controlled trial will determine whether a novel technologically-enabled, behaviorally-targeted, pharmacist-based intervention improves adherence to medications for chronic diseases and disease control. This study will also provide generalizable information about how to tailor patient interventions and integrate advance communication technologies into clinical practice.
Supplementary Material
Pragmatic clinical trial evaluating a strategy to improve medication adherence
Subjects are non-adherent to chronic medications and have poor disease control
Intervention uses telephonic pharmacist consultation, texts, and mailed reports
Trial’s primary outcome is medication adherence
Acknowledgments
This research was supported by a grant from NHLBI to Brigham and Women’s Hospital (R01 HL 117918). We wish to thank several individuals for their assistance with the trial. Tara Raj, Julianne McDonough and Lajja Patel for patient recruitment, Leilani Hernandez for data management, William Keough for creating the electronic health record tools necessary to conduct the study, and Kelly O’Keefe for her assistance setting up the study management system.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Avorn J, Monette J, Lacour A, et al. Persistence of use of lipid-lowering medications: a cross-national study. JAMA. 1998;279(18):1458–1462. doi: 10.1001/jama.279.18.1458. [DOI] [PubMed] [Google Scholar]
- 2.Benner JS, Glynn RJ, Mogun H, Neumann PJ, Weinstein MC, Avorn J. Long-term persistence in use of statin therapy in elderly patients. JAMA. 2002;288(4):455–461. doi: 10.1001/jama.288.4.455. [DOI] [PubMed] [Google Scholar]
- 3.Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA. 2002;288(4):462–467. doi: 10.1001/jama.288.4.462. [DOI] [PubMed] [Google Scholar]
- 4.Choudhry NK, Setoguchi S, Levin R, Winkelmayer WC, Shrank WH. Trends in adherence to secondary prevention medications in elderly post-myocardial infarction patients. Pharmacoepidemiol Drug Saf. 2008;17(12):1189–1196. doi: 10.1002/pds.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.IMS Institute for Healthcare Informatics Avoidable Costs in US Health Care. [Accessed September 10, 2013];2013 Available at: http://www.imshealth.com/deployedfiles/imshealth/Global/Content/Corporate/IMS%20Institute/RUOM-2013/IHII_Responsible_Use_Medicines_2013.pdf.
- 6.Benjamin RM. Medication adherence: helping patients take their medicines as directed. Public Health Rep Wash DC 1974. 2012;127(1):2–3. doi: 10.1177/003335491212700102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 8.Adherence to long-term therapies. World Health Organization; 2003. [Google Scholar]
- 9.Viswanathan M, Golin CE, Jones CD, et al. Interventions to improve adherence to self-administered medications for chronic diseases in the United States: a systematic review. Ann Intern Med. 2012;157(11):785–795. doi: 10.7326/0003-4819-157-11-201212040-00538. [DOI] [PubMed] [Google Scholar]
- 10.Thakkar J, Kurup R, Laba T-L, et al. Mobile Telephone Text Messaging for Medication Adherence in Chronic Disease: A Meta-analysis. JAMA Intern Med. 2016;176(3):340–349. doi: 10.1001/jamainternmed.2015.7667. [DOI] [PubMed] [Google Scholar]
- 11.Wald DS, Butt S, Bestwick JP. One-way versus two-way text messaging on improving medication adherence: meta-analysis of randomized trials. Am J Med. 2015;128(10):1139.e1–5. doi: 10.1016/j.amjmed.2015.05.035. [DOI] [PubMed] [Google Scholar]
- 12.Barker-Collo S, Krishnamurthi R, Witt E, et al. Improving Adherence to Secondary Stroke Prevention Strategies Through Motivational Interviewing: Randomized Controlled Trial. Stroke J Cereb Circ. 2015 Oct; doi: 10.1161/STROKEAHA.115.011003. [DOI] [PubMed] [Google Scholar]
- 13.Hedegaard U, Kjeldsen LJ, Pottegård A, et al. Improving Medication Adherence in Patients with Hypertension: A Randomized Trial. Am J Med. 2015 Aug; doi: 10.1016/j.amjmed.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 14.Pfaeffli Dale L, Whittaker R, Jiang Y, Stewart R, Rolleston A, Maddison R. Text Message and Internet Support for Coronary Heart Disease Self-Management: Results From the Text4Heart Randomized Controlled Trial. J Med Internet Res. 2015;17(10):e237. doi: 10.2196/jmir.4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stewart K, George J, Mc Namara KP, et al. A multifaceted pharmacist intervention to improve antihypertensive adherence: a cluster-randomized, controlled trial (HAPPy trial) J Clin Pharm Ther. 2014;39(5):527–534. doi: 10.1111/jcpt.12185. [DOI] [PubMed] [Google Scholar]
- 16.Cutrona SL, Choudhry NK, Fischer MA, et al. Targeting cardiovascular medication adherence interventions. J Am Pharm Assoc JAPhA. 2012;52(3):381–397. doi: 10.1331/JAPhA.2012.10211. [DOI] [PubMed] [Google Scholar]
- 17.Nieuwlaat R, Wilczynski N, Navarro T, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2014;11:CD000011. doi: 10.1002/14651858.CD000011.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Diabetes Association. 5. Glycemic Targets. Diabetes Care. 2016;39(Suppl 1):S39–46. doi: 10.2337/dc16-S008. [DOI] [PubMed] [Google Scholar]
- 19.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311(5):507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 20.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S1–45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 21.Choudhry NK, Shrank WH, Levin RL, et al. Measuring concurrent adherence to multiple related medications. Am J Manag Care. 2009;15(7):457–464. [PMC free article] [PubMed] [Google Scholar]
- 22.Hibbard JH, Stockard J, Mahoney ER, Tusler M. Development of the Patient Activation Measure (PAM): conceptualizing and measuring activation in patients and consumers. Health Serv Res. 2004;39(4 Pt 1):1005–1026. doi: 10.1111/j.1475-6773.2004.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hibbard JH, Mahoney ER, Stockard J, Tusler M. Development and testing of a short form of the patient activation measure. Health Serv Res. 2005;40(6 Pt 1):1918–1930. doi: 10.1111/j.1475-6773.2005.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rollnick S, Kinnersley P, Stott N. Methods of helping patients with behaviour change. BMJ. 1993;307(6897):188–190. doi: 10.1136/bmj.307.6897.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heather N, Rollnick S, Bell A, Richmond R. Effects of brief counselling among male heavy drinkers identified on general hospital wards. Drug Alcohol Rev. 1996;15(1):29–38. doi: 10.1080/09595239600185641. [DOI] [PubMed] [Google Scholar]
- 26.Samet JH, Rollnick S, Barnes H. Beyond CAGE. A brief clinical approach after detection of substance abuse. Arch Intern Med. 1996;156(20):2287–2293. doi: 10.1001/archinte.156.20.2287. [DOI] [PubMed] [Google Scholar]
- 27.Wilson IB, Fowler FJ, Cosenza CA, Michaud J, Bentkover J, Rana A, Kogelman L, Rogers WH. Cognitive and field testing of a new set of medication adherence self-report items for HIV care. AIDS Behav. 2014;18:2349–2358. doi: 10.1007/s10461-013-0610-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Newgard CD, Lewis RJ. Missing Data: How to Best Account for What Is Not Known. JAMA. 2015;314(9):940–941. doi: 10.1001/jama.2015.10516. [DOI] [PubMed] [Google Scholar]
- 29.Glynn RJ, Brookhart MA, Stedman M, Avorn J, Solomon DH. Design of cluster-randomized trials of quality improvement interventions aimed at medical care providers. Med Care. 2007;45(10 Supl 2):S38–43. doi: 10.1097/MLR.0b013e318070c0a0. [DOI] [PubMed] [Google Scholar]
- 30.Choudhry NK, Shrank WH. Implementing randomized effectiveness trials in large insurance systems. J Clin Epidemiol. 2013;66(8 Suppl):S5–11. doi: 10.1016/j.jclinepi.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 31.Choudhry NK, Shrank WH. Four-dollar generics--increased accessibility, impaired quality assurance. N Engl J Med. 2010;363(20):1885–1887. doi: 10.1056/NEJMp1006189. [DOI] [PubMed] [Google Scholar]
- 32.Lauffenburger JC, Balasubramanian A, Farley JF, et al. Completeness of prescription information in US commercial claims databases. Pharmacoepidemiol Drug Saf. 2013;22(8):899–906. doi: 10.1002/pds.3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hansen RA, Kim MM, Song L, Tu W, Wu J, Murray MD. Comparison of methods to assess medication adherence and classify nonadherence. Ann Pharmacother. 2009;43(3):413–422. doi: 10.1345/aph.1L496. [DOI] [PubMed] [Google Scholar]
- 34.Lau HS, de Boer A, Beuning KS, Porsius A. Validation of pharmacy records in drug exposure assessment. J Clin Epidemiol. 1997;50(5):619–625. doi: 10.1016/s0895-4356(97)00040-1. [DOI] [PubMed] [Google Scholar]
- 35.Schmittdiel JA, Nichols GA, Dyer W, Steiner JF, Karter AJ, Raebel MA. Health care system-level factors associated with performance on Medicare STAR adherence metrics in a large, integrated delivery system. Med Care. 2015;53(4):332–337. doi: 10.1097/MLR.0000000000000328. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.