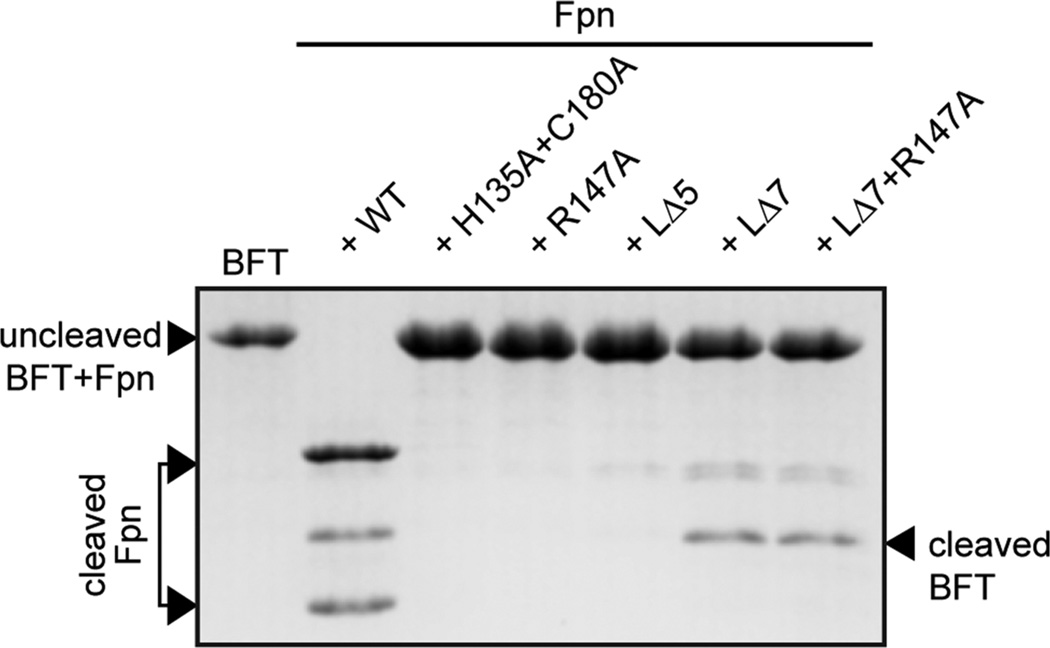
Figure 4.
Testing Fpn active site and loop mutants as proteases of the B. fragilis toxin, BFT. Wild-type Fpn cleavage of BFT results in a 17 kDa band on a SDS–PAGE gel. The active site mutant (FpnH135A+C180A), the cleavage site mutant (FpnR147A), and the loop mutant (FpnLΔ5) do not process BFT after incubation at 37 °C for 45 min. A loopless mutant of Fpn (FpnLΔ7) and a loopless mutant of Fpn harboring a substitution of the arginine residue at the loop cleavage site (FpnLΔ7+R147A) cleave BFT.