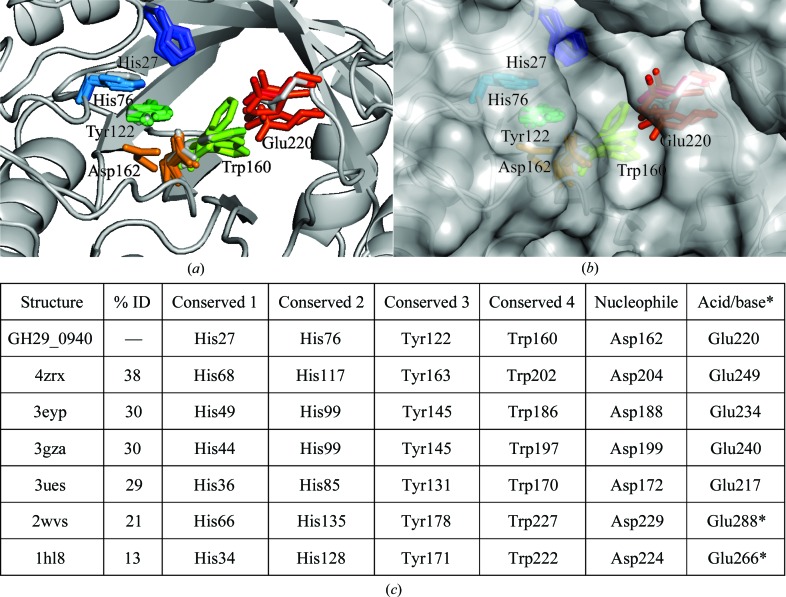
Figure 4.
A catalytic domain comparison of residues found to be highly conserved (as determined by a structural alignment; Fig. 1 ▸) in the active pocket of GH29_0940 and its related structures. Six (out of 21) conserved residues are located in the active site. (a, b) A cartoon representation of a superposition of related GH29 structures (PDB entries 4zrx, 3eyp, 3ues, 3gza, 2wvs and 1hl8), showing the conserved residues as coloured sticks (GH29_0940 in grey) and a grey surface representation. Residues are numbered according to GH29_0940 numbering. (c) A table detailing the position of each conserved residue in each structure and the percentage sequence identity (residues marked with an asterisk are not found in the same structural position in the sequence alignment and hence are conservative but not identical). The structures were superimposed using Coot (Emsley et al., 2010 ▸) and all figures were produced using PyMOL v.1.3 (http://www.pymol.org; Schrödinger).