Figure 1.
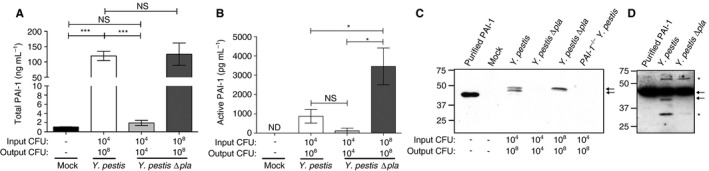
Plasminogen activator inhibitor‐1 (PAI‐1) is cleaved and inactivated by the Pla protease in vivo. (A, B) Quantification of (A) total PAI‐1 and (B) active PAI‐1 by ELISA recovered by bronchoalveolar lavage (BAL) of C57BL/6 mouse lungs 48 h after inoculation with phosphate‐buffered saline (mock), 104 colony‐forming units (CFUs) of Yersinia pestis, 104 CFUs of Y. pestis ∆pla, or 108 CFUs of Y. pestis ∆pla. Data from two independent experiments are combined (n = 10 for each group); error bars represent standard errors of the mean (SEMs), and significance was determined by one‐way anova with Bonferroni's multiple comparison (*P ≤ 0.05; ***P ≤ 0.001). (C, D) Immunoblot analysis of PAI‐1 recovered by BAL (C) or PAI‐1 cleaved in vitro (D). Blots are representative of three independent experiments. Arrows represent full‐length PAI‐1 protein and cleavage product; asterisks indicate non‐specific bands. Numbers to the left of the blot indicate molecular masses in kilodaltons. Note that purified, recombinant PAI‐1 migrates at a lower molecular mass than PAI‐1 isolated from mouse BAL fluid, owing to the lack of post‐translational modifications on the protein. The input CFU and output CFU are included below each figure to denote the number of bacteria inoculated and the number at the time of assessment, respectively. ND, not detectable; NS, not significant.
