Abstract
Primary mucinous cystadenocarcinoma (MCA) of the breast is a rare but pathologically distinct breast tumor. There have been some case reports on primary MCA of the breast; however, they have all focused on pathologic findings. Here, we report the radiologic findings of two cases of MCA along with a review of the literature. Breast MCA shows a circumscribed mass with some calcifications on mammography, an intracystic solid mass without increased vascularity or a vascular stalk on ultrasound, and a heterogeneously enhancing mass within a rim-enhancing cyst with intermediate signal intensity on T2-weighted magnetic resonance imaging. These radiologic findings and the presence of mucin in the percutaneous biopsy specimen should suggest the possibility of MCA in the differential diagnosis of a breast tumor.
Keywords: Breast, Magnetic resonance imaging, Mucinous cystadenocarcinoma, Ultrasound
INTRODUCTION
Mucinous cystadenocarcinoma (MCA) is a very rare breast tumor. To our knowledge, only 18 cases of MCA have been published in the English literature, mostly focusing on the pathologic findings [1,2,3,4,5,6,7,8,9]. MCA is a pathologically distinct disease entity; however, radiologic findings have not yet been described in precise detail. Here, we report mammographic, ultrasound (US), and magnetic resonance imaging (MRI) findings of primary breast MCA in two patients, and review the literature focusing on imaging findings.
CASE REPORTS
Case 1
A 59-year-old woman presented with a mass in her left breast detected on screening mammography. She was postmenopausal and did not have a family history of breast cancer or other malignancy according to her medical records. Physical examination revealed a 1.5 cm mass in the upper outer quadrant of the left breast, 5 cm from the nipple.
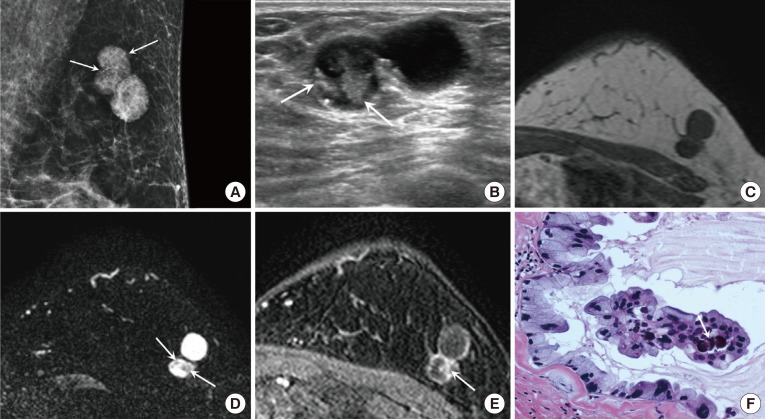
Mammography showed a lobular-shaped circumscribed hyperdense mass, containing round or punctate microcalcifications (Figure 1A).
Figure 1. A 59-year-old female with mucinous cystadenocarcinoma (MCA) in the left breast. (A) Mediolateral oblique view of mammography shows a 2.8 cm sized lobular-shaped circumscribed hyperdense mass containing round or punctate microcalcifications (arrows) in the upper outer quadrant of the left breast. (B) On ultrasound, the MCA appears as a circumscribed complex cystic and solid mass. The solid portion of the mass (arrows) shows an irregular shape and isoechogenicity, with multiple echogenic dots corresponding to the microcalcifications seen on mammography. On magnetic resonance imaging, the solid portion shows low signal intensity (SI) on T1 weighted imaging (WI) (C) and intermediate SI on T2-WI (arrows) (D). The cystic portion shows rim-enhancement and the solid portion (arrow) shows nodular enhancement on dynamic contrast-enhanced image with persistent enhancement kinetics (E). (F) The tumor is composed of multiple cysts distended by mucinous secretion and the cysts are lined with tall columnar cells with intracytoplasmic Most nuclei are basally located and somewhat bland, however some nuclei show more atypia. Note the psammomatous calcification (arrow) in the papillary core (H&E stain, ×400).
On US, the mass was located at the far periphery of the left upper outer breast and appeared as a circumscribed complex cystic and solid mass. The solid portion of the mass showed an irregular shape and isoechogenicity, with multiple echogenic dots corresponding to the microcalcifications seen on mammography (Figure 1B).
MRI showed a circumscribed lobular complex cystic and solid mass. The solid portion showed low signal intensity (SI) on T1 weighted imaging (WI) (Figure 1C) and intermediate SI on T2 WI (Figure 1D). On dynamic contrast-enhanced images, the mass showed rim enhancement and nodular enhancement of the solid portion with persistent enhancement kinetics (Figure 1E).
The result of percutaneous core needle biopsy using a 14-gauge gun was intraductal carcinoma with mucin spillage. The differential diagnosis based on the percutaneous biopsy result was mucocele-like lesions with ductal carcinoma in situ or mucinous carcinoma with ductal carcinoma in situ.
After partial mastectomy, a 2.0 cm MCA with Bloom-Richardson grade I was diagnosed. The tumor was composed of multiple cysts distended by mucinous secretions. The cysts were lined with tall columnar cells with intracytoplasmic mucin vacuoles. Most nuclei were basally located and somewhat bland; however, some nuclei showed more atypia (Figure 1F). The tumor cells were negative for estrogen and progesterone receptors but were positive for human epidermal growth factor receptor 2 (HER2; 3+).
Case 2
A 50-year-old woman presented with a palpable mass in her left breast. She was premenopausal and had no family history of breast cancer or other malignancy according to her medical records.
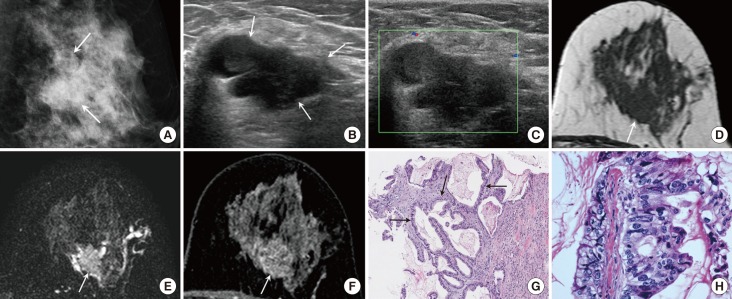
A lobular-shaped isodense mass was detected on mammography. The mass showed obscured margins and had a few faint microcalcifications (Figure 2A).
Figure 2. A 50-year-old female with mucinous cystadenocarcinoma in the left breast. (A) Mediolateral oblique view of mammography shows a 2.8 cm sized lobular-shaped isodense mass with obscured margin and a few faint microcalcifications in the left mid outer breast (arrows). (B) A multilobulated complex cystic and solid mass is seen on ultrasound (US). The solid portion of the mass (arrows) is hypoechoic and the margin is partially indistinct. (C) Vascularity is not increased and a vascular stalk is not detected on Doppler US. On magnetic resonance imaging, a cystic and solid mass is shown. The solid portion (arrow) has an irregular shape and the margin shows hypointensity on T1 weighted imaging (WI) (D), intermediate signal intensity on T2-WI (E), and heterogeneous enhancement with persistent enhancement kinetics (F). (G) Core biopsy shows multilocular cystic lesion containing extracellular mucin. The cyst wall is lined with tall columnar mucinous epithelium (arrows) (H&E stain, ×100). (H) Mucinous epithelium shows marked atypia and pleomorphism. There are frequent mitotic figures (H&E stain, ×400).
US showed a multilobulated complex cystic and solid mass. The solid portion of the mass showed hypoechogenicity and had a partially indistinct margin (Figure 2B). On Doppler US, no increased vascularity or vascular stalk was noted (Figure 2C).
On MRI, the mass in the left mid outer breast was shown to be cystic and solid. The solid portion showed an irregular shape and margin, had hypointensity on T1 WI (Figure 2D), intermediate SI on T2 WI (Figure 2E), and heterogeneous enhancement with persistent enhancement kinetics (Figure 2F). The cystic portion showed a circumscribed margin and rim enhancement.
Percutaneous core needle biopsy using a 14-gauge gun found cystically dilated atypical mucinous glands, and MCA was suspected.
After a left partial mastectomy, a 2.2 cm MCA with Bloom-Richardson grade III was diagnosed (Figure 2G, H). Multiple variable sized cysts were filled with mucin. In this case, the mucinous epithelium showed marked atypia and pleomorphism. There were frequent mitotic figures. The tumor cells were negative for estrogen and progesterone receptors and were also negative for HER2.
DISCUSSION
MCA of the breast is an extremely rare tumor. It is homologous to MCA of the ovary and pancreas [10]. MCA has distinct pathologic features compared with mucinous carcinoma. MCA is characterized by cystic lesion containing mucin lined by columnar cells with abundant intracytoplasmic mucin or mucin-depleted columnar cells, while mucinous carcinoma is characterized by clusters of generally small and uniform cells floating in large amount of extracellular mucin [11]. Previous reports have mostly focused on pathologic findings, with only a few reports briefly describing radiologic findings, providing few details. We reviewed the reported radiologic findings, which are summarized in Table 1.
Table 1. Publications on the primary mucinous cystadenocarcinoma of the breast.
| Author (year) | No. of cases | Mammographic findings | Ultrasonographic findings | ER/PR/HER2 |
|---|---|---|---|---|
| Lin et al. (2013) [8] | 1 | NA | Well-circumscribed and lobulated cystic-solid mass | –/–/– |
| Deng et al. (2012) [4] | 1 | Patchy irregular calcifications within the masses | Irregularly shape lesion | –/–/– |
| Li et al. (2012) [6] | 1 | Well-defined, medium to high density, multilobulated mass | NA | –/–/– |
| Sentani et al. (2012) [7] | 1 | Well-defined, medium to high density, lobulated mass | Circumscribed, isoechoic to hypoechonic lesion | –/–/NA |
| Kim et al. (2012) [5] | 1 | Focal asymmetry | Hypoechoic mass with a speculated margin | –/–/– |
| Rakıcı et al. (2009) [3] | 1 | Well-defined multilobular mass | NA | +/–/– |
| Lee and Chaung (2008) [9] | 1 | Round, isodense, circumscribed mass | Circumscribed lesion with complex hypoechoic to isoechoic contents | –/–/– |
| Chen et al. (2004) [2] | 1 | Well-defined, medium to high density, multilobulated mass | Circumscribed, isoechoic to hypoechoic lesion | –/–/– |
| Honma et al. (2003) [1] | 1 | NA | NA | –/–/– |
ER=estrogen receptor; PR=progesterone receptor; HER2=human epidermal growth factor receptor 2; NA=not available.
Mammographic findings of MCA have been described in several case reports, and the most common finding was a well-defined multilobular mass with medium to high density [2,3,6,7,9]. One case had irregular macrocalcifications [4]. Our cases showed lobular shaped masses with relatively circumscribed margins and round to punctate internal microcalcifications.
Reported US findings of primary breast MCA are mostly circumscribed isoechoic to hypoechoic masses [2,7,9]; occasionally, irregular masses or cystic and solid masses have also been reported [4,5,8]. Both of our cases showed complex cystic and solid masses on US. One case showed a circumscribed complex cystic mass with a small solid portion, whereas the other showed a more solid portion with an irregular shape and indistinct margins.
MRI findings have not been described previously. In our cases, both tumors showed cystic and solid masses and the cystic portion of the masses showed a circumscribed margin and thin rim enhancement. The intracystic solid portion of the tumor showed nodular enhancement and a large irregular solid mass with heterogeneous enhancement. Both showed persistent enhancement kinetics on dynamic enhancement images and intermediate SI on T2 WI.
In our cases, from the US findings of a complex cystic and solid mass, we considered the possibility of papillary neoplasm first. However, a vascular stalk or increased vascularity, which is a characteristic finding of papillary neoplasm, was not detected [12]. On MRI, our cases also showed a cystic portion with rim enhancement and an intracystic solid portion, which may suggest a papillary tumor. However, the enhancement kinetics of our cases was persistent, while papillary neoplasms commonly show washout enhancement kinetics.
In the first case, the percutaneous biopsy result was intraductal carcinoma with mucin spillage, suggesting the possibility of mucinous carcinoma. However, US showed a cystic mass with a small intracystic nodule with microcalcifications, which is not the typical finding for mucinous carcinoma, namely, an isoechoic solid mass with heterogeneous echotexture and a circumscribed or microlobulated margin [13]. Mucinous carcinoma can also show cystic changes within the solid tumor because of the mucin pool [13], but our case showed a large cyst with a small nodule similar to intracystic papilloma rather than cystic changes within the solid tumor [13]. Moreover, the microcalcifications on mammography were small and round rather than the coarse and heterogeneous microcalcifications commonly detected in mucinous carcinoma. On MRI, the solid portion of the tumor showed an intermediate signal on T2 WI that was not as bright as that usually seen in the typical mucinous carcinoma [14].
Mucocele-like lesions also show mucinous material in percutaneous biopsy samples, and they can have various shapes of calcifications on mammography. However, they are usually benign cystic masses, including simple cysts, complicated cysts, and clustered microcysts, rather than complex cystic and solid masses, unless they are associated with atypical ductal hyperplasia or malignancy [15].
In conclusion, MCA shows a circumscribed mass with some lower suspicion microcalcifications on mammography, and shows an intracystic solid mass without increased vascularity or a vascular stalk on US. On MRI, the solid portion shows intermediate SI on T2 WI and heterogeneous enhancement, and the cystic portion shows rim enhancement. When the above-described findings are detected during radiologic exams, and mucinous material is present in the biopsy specimen, MCA can be considered as a differential diagnosis.
Footnotes
CONFLICT OF INTEREST: The authors declare that they have no competing interests.
References
- 1.Honma N, Sakamoto G, Ikenaga M, Kuroiwa K, Younes M, Takubo K. Mucinous cystadenocarcinoma of the breast: a case report and review of the literature. Arch Pathol Lab Med. 2003;127:1031–1033. doi: 10.5858/2003-127-1031-MCOTBA. [DOI] [PubMed] [Google Scholar]
- 2.Chen WY, Chen CS, Chen HC, Hung YJ, Chu JS. Mucinous cystadenocarcinoma of the breast coexisting with infiltrating ductal carcinoma. Pathol Int. 2004;54:781–786. doi: 10.1111/j.1440-1827.2004.01755.x. [DOI] [PubMed] [Google Scholar]
- 3.Rakıcı S, Gönüllü G, Gürsel SB, Yıldız L, Bayrak IK, Yücel I. Mucinous cystadenocarcinoma of the breast with estrogen receptor expression: a case report and review of the literature. Case Rep Oncol. 2009;2:210–216. doi: 10.1159/000253866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deng Y, Xue D, Wang X, Xu S, Ao Q, Hu Z, et al. Mucinous cystadenocarcinoma of the breast with a basal-like immunophenotype. Pathol Int. 2012;62:429–432. doi: 10.1111/j.1440-1827.2012.02810.x. [DOI] [PubMed] [Google Scholar]
- 5.Kim SE, Park JH, Hong S, Koo JS, Jeong J, Jung WH. Primary mucinous cystadenocarcinoma of the breast: cytologic finding and expression of MUC5 are different from mucinous carcinoma. Korean J Pathol. 2012;46:611–616. doi: 10.4132/KoreanJPathol.2012.46.6.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X, Peng J, Zhang Z, Zhang Y. Mammary mucinous cystadenocarcinoma. Breast J. 2012;18:282–283. doi: 10.1111/j.1524-4741.2012.01238.x. [DOI] [PubMed] [Google Scholar]
- 7.Sentani K, Tashiro T, Uraoka N, Aosaki Y, Yano S, Takaeko F, et al. Primary mammary mucinous cystadenocarcinoma: cytological and histological findings. Diagn Cytopathol. 2012;40:624–628. doi: 10.1002/dc.21638. [DOI] [PubMed] [Google Scholar]
- 8.Lin DL, Hu JL, Shao SH, Sun DM, Wang JG. Primary mucinous cystadenocarcinoma of the breast with endocervical-like mucinous epithelium. Breast Care (Basel) 2013;8:445–447. doi: 10.1159/000357657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee SH, Chaung CR. Mucinous metaplasia of breast carcinoma with macrocystic transformation resembling ovarian mucinous cystadenocarcinoma in a case of synchronous bilateral infiltrating ductal carcinoma. Pathol Int. 2008;58:601–605. doi: 10.1111/j.1440-1827.2008.02278.x. [DOI] [PubMed] [Google Scholar]
- 10.Witherspoon LE, Oxenhandler RW. A rare tumor: mucinous cystadenocarcinoma of the breast. Am Surg. 2015;81:E106–E108. [PubMed] [Google Scholar]
- 11.Ellis IO, Cornelisse CJ, Schnitt SJ, Sasco AJ, Sastre-Garau X, Kaaks R, et al. Mucin producing carcinomas. In: Tavassoli FA, Devilee P, editors. Pathology and Genetics of Tumours of the Breast and Female Genital Organs. Lyon: IARC; 2003. pp. 30–32. [Google Scholar]
- 12.Rodríguez MC, Secades AL, Angulo JM. Best cases from the AFIP: intracystic papillary carcinoma of the breast. Radiographics. 2010;30:2021–2027. doi: 10.1148/rg.307105003. [DOI] [PubMed] [Google Scholar]
- 13.Lam WW, Chu WC, Tse GM, Ma TK. Sonographic appearance of mucinous carcinoma of the breast. AJR Am J Roentgenol. 2004;182:1069–1074. doi: 10.2214/ajr.182.4.1821069. [DOI] [PubMed] [Google Scholar]
- 14.Monzawa S, Yokokawa M, Sakuma T, Takao S, Hirokaga K, Hanioka K, et al. Mucinous carcinoma of the breast: MRI features of pure and mixed forms with histopathologic correlation. AJR Am J Roentgenol. 2009;192:W125–W131. doi: 10.2214/AJR.07.4021. [DOI] [PubMed] [Google Scholar]
- 15.Kim SM, Kim HH, Kang DK, Shin HJ, Cho N, Park JM, et al. Mucocele-like tumors of the breast as cystic lesions: sonographic-pathologic correlation. AJR Am J Roentgenol. 2011;196:1424–1430. doi: 10.2214/AJR.10.5028. [DOI] [PubMed] [Google Scholar]