Abstract
Background
The goal of this study was to perform a systematic review to examine the efficacy and safety of various salvage therapy regimens on patients with relapsed/refractory PTCL.
Method
The electronic searches were performed using PubMed, Cochrane Library, EMBASE, and Web of Science from inception through June 2015, with search terms related to relapsed/refractory PTCL, salvage chemotherapy regimens, and clinical trials. An eligible study met the following inclusion criteria: (1) Patients had refractory or relapsed PTCL; (2) drug regimens were used for salvage therapy; (3) the study was a clinical trial; (4) the study reported on a series of at least 10 patients of PTCL.
Results
Of 35 records identified, a total of 14 studies were eligible for systematic reviews, and 12 different salvage regimens were investigated. A total of 618 relapsed/refractory PTCL patients were identified. The ORRs ranged from 22% for those treated with lenalidomide to 86% for those with brentuximab vedotin. By the three most frequent subtypes, the ORRs ranged from 14.2% to 71.5% for patients with the PTCL-NOS subtype, 8% to 54% for AITL subtypes, and 24% to 86% for the ALCL subtype. The medians of DOR, PFS, and OS ranged from 2.5 to 16.6 months, 2.6 to 13.3 months, and 3.6 to 14.5 months, respectively. The most frequently reported grade 3 or 4 adverse events (AEs) were hematological AEs, such as neutropenia and thrombocytopenia.
Conclusion
The efficacy of salvage therapy regimens is highly diverse for patients with relapsed/refractory PTCL; this heterogeneity in therapeutic effects might be due to the diversity in mechanisms, PTCL subtype distribution, and/or numbers/profiles of prior therapy. Comparative studies with matched pair analysis are warranted for more evidence of the salvage treatment effect on relapsed or heavily pretreated patients with PTCL.
Introduction
Peripheral T-cell lymphomas (PTCL) are a group of relatively rare, clinically and biologically heterogeneous lymphoproliferative disorders that develop in mature blood cell called “T cells” and “natural killer (NK) cells [1], which account for 10–15% of all non-Hodgkin’s lymphomas (NHL) in the Western population [2]. Generally, PTCLs are significantly rarer and more difficult to treat when compared to their B-cell counterparts [3], either due to the paucity of large trials carrying the evidence to suggest specific therapeutic approaches or due to the biology of the disease. The 2008 World Health Organization classification system contains 22 different T-cell lymphoma subgroups, which are distinct regarding pathology, clinical presentation, response to therapy, and expression of surface markers [2,4,5]. According to the International T-Cell Lymphoma Project [6], the most common subtypes are PTCL-not otherwise specified (PTCL-NOS) (25.9%), angioimmunoblastic T-cell lymphoma (AITL) (18.5%), and anaplastic large cell lymphoma (ALCL) (12.1%), which might be positive or negative for anaplastic lymphoma kinase (ALK). However, the distributions of the various lymphoma subtypes are geographically diverse; for example, PTCL-NOS is the most common subtype in North American and Europe, while adult T-cell leukemia/lymphoma (ATLL) is most common in Asia [6].
Patients with PTCL are characterized by poor treatment outcomes with conventional chemotherapy and no established standards of care for patients with relapsed and refractory settings [7]. Standard first-line therapy consists of CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) or a CHOP-like regimen. Nevertheless, therapeutic responses to this approach have been neither appropriate nor durable [8]. According to the International T-Cell Lymphoma Project, the 5-year overall survival rate is poor for most subtypes: 32% for PTCL-NOS and AITL, and 14% for ATLL [6]. With conventional chemotherapy alone, a population-based cancer registry study showed that median overall survival (OS) is only 6.5 months for patients after first relapse or progression of PTCL [9]. The generally poor outcomes observed in PTCL patients emphasize the urgent need for alternative therapy [10]. Moreover, due to its rarity and the heterogeneity of subtypes, randomized controlled trials comparing different treatment approaches for PTCL are very limited [5]. Several novel approaches have been evaluated in single-arm phase I and II studies, mainly in patients with relapsed/refractory disease who had particularly poor prognoses [5].
In the last 5 years, several therapeutic agents with novel mechanisms of action have been approved by the US Food and Drug Administration (FDA) for patients with relapsed/refractory PTCL [11,12], including pralatrexate [13], romidespsin [14], brentuximab vedotin [15], and belinostat [16]. According to the National Comprehensive Cancer Network (NCCN) guidelines [17], FDA-approved agents, promising single agents, and some combination chemotherapies were advocated as the second-line therapy for relapse/refractory PTCL, both for candidates and non-candidates for stem cell transplantation (SCT). To the extent of our knowledge, there are no systematic reviews for the salvage treatment regimens focused on relapsed/refractory PTCL, as only narrative reviews exist [5,7]. In order to capture the changing landscape of the salvage treatment for relapsed/refractory PTCL, we conducted a systematic review regarding the efficacy and safety of salvage treatment regimens among patients with relapsed/refractory PTCL.
Methods
Literature Search Strategy
According to NCCN guidelines (Version 5, 2014) [17] for PTCL, the suggested treatment regimens of second-line therapy are as follows (in alphabet order): (1) single-agent therapy: alemtuzumab, belinostat, bortezomib, brentuximab vedotin, cyclosporine, gemcitabine, pralatrexate, romidepsin; (2) combination therapy: DHAP (dexamethasone, cytarabine, cisplatin), dose-adjusted EPOCH (etoposide, prednisolone, vincristine, cyclophosphamide, doxorubicin), ESHAP (etoposide, methylprednisolone, cytarabine, cisplatin), GDP (gemcitabine, dexamethasone, cisplatin), GemOx (gemcitabine, oxaliplatin), ICE (ifosfamide, carboplatin, etoposide), MINE (mesna, ifosfamide, mitoxantrone, etoposide). The electronic searches were performed using PubMed, Cochrane Library, EMBASE and Web of Science from their date of inception to June 12, 2015. To identify specific and relevant studies, we used a search strategy based on patient population (relapsed/refractory PTCL), treatments (NCCN guide suggested regimens and other salvage chemotherapy), and study designs (phase II clinical trials) (Tables A and B in S1 File). The keywords in the extensive electronic literature search, such as “Peripheral T-cell Lymphoma,” “Relapsed or Refractory,” “Salvage therapy,” and “Clinical Trial,” were transformed into MeSH terms. The references from the NCCN guidelines for relapsed/refractory PTCL were also accessed for eligibility in the review process (as another resource). All identified articles were systematically assessed using the inclusion and exclusion criteria.
Selection Criteria
In this systematic review, an eligible study met the following inclusion criteria: (1) Patients had refractory or relapsed PTCL; (2) Drug regimens were used for salvage therapy or relevant to those previously defined; (3) the study was a clinical trial (phase II); (4) the study reported on a series of at least 10 patients of PTCL to prevent bias arising from small sample populations.
Exclusion criteria were as follows: (1) Patients were irrelevant to any subtype of PTCL (cutaneous T-cell lymphoma [CTCL] are excluded) or were not treated previously (not relapsed or refractory); (2) Drug regimen was used as front therapy, not salvage therapy; (3) Retrospective studies, phase I clinical trial, review paper, comment, letter, abstract, editorial; (4) Patient number of PTCL was less than 10; (5) Absence of efficacy outcome such as overall response rate (ORR).
Rather than conduct a narrow search for specific chemotherapy combinations, we separately searched each single agent within a given combination in order to generate the broadest possible search results that would include all possible treatment combinations in which that agent appeared. For example, dexamethasone, a common agent in chemotherapy, was searched alone so that both DHAP and GDP appeared as possible combinations. However, if only a single agent was found alone in some studies, these papers were also included in the systematic review process for eligibility.
Quality Assessment
The common quality assessment tools suggested for randomized controlled trials (RCTs) were “Cochrane’s risk of bias checklist” and the “SIGN50 RCT checklist”, and another tool for non-randomized studies of interventions was also recommended [18]. However, since the studies we included were more likely single-armed, these three tools might not have been able to adequately evaluate the study quality of these selected papers. Here, we proposed a modified 18-item checklist that was often used in quality appraisals of case-series or one-group studies [19]. The minimum number of satisfied checklist items necessary for a study to be classified as high quality was determined to be 10.
Data extraction and critical appraisal
The primary outcome measure was ORR, and secondary outcome measures included duration of response (DOR), progression-free survival (PFS), and OS. The median of these durations (including range or 95% confidence interval [CI]) was extracted. With regard to safety outcomes, World Health Organization (WHO) grade 3 or 4 hematological and non-hematological adverse events (AEs) were extracted respectively. Moreover, extracted data also included type of study, PTCL subtype, prior therapy (number of prior therapies, autologous hematopoietic stem cell transplantation [auto-HSCT]), demographic characteristics of patients (age, gender, and ethnics), and median follow-up time. Quality-control procedures for the data extraction comprised of verification of all extracted data with their original sources by a second researcher. The study selection, quality assessment, and data extraction were performed independently by two reviewers using a standardized form. The discrepancies between the two reviewers were resolved through discussion and consensus.
Data Analysis
A narrative review of these studies was presented. Data on characteristics of studies were presented, if available. The ORR and 95% CI across various studies were graphically presented, and the corresponding plot was conducted by Comprehensive Meta-Analysis, Version 2 (Biostat Inc., Englewood, NJ, USA).
Results
Screening process and quality of included studies
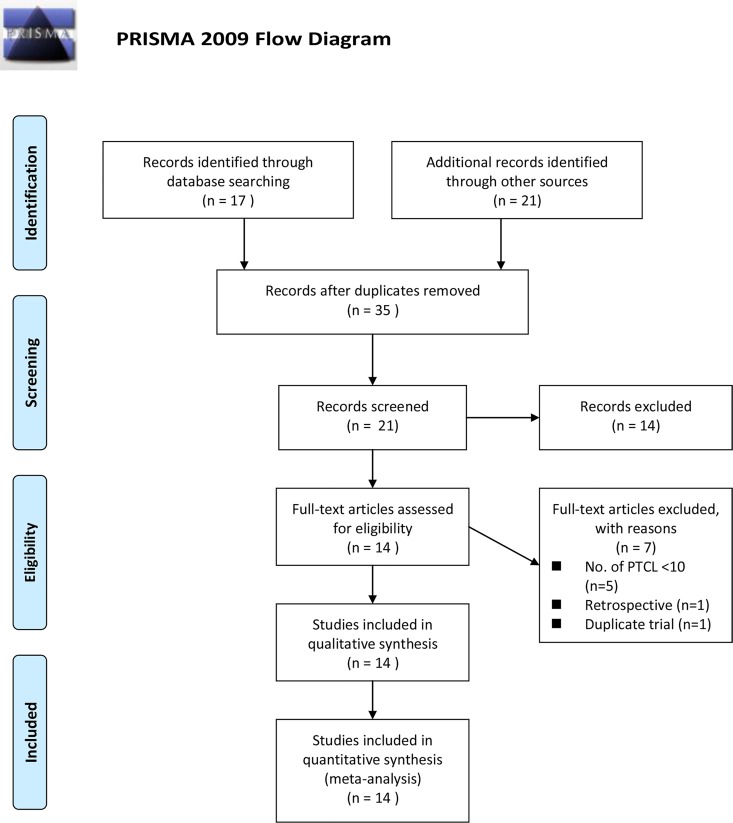
A total of 35 records were selected for abstract screening (databases = 17, other resources = 21, duplicate records = 3). A total of 21 records (databases = 16; other resources = 5) were identified for full-text screening. A total of 14 records were excluded either because only their abstracts were available (n = 3), the patients did not pertain to any subtype of PTCL or relapsed/refractory setting (n = 6), or the therapies (n = 3) or study designs (n = 2) were irrelevant. Out of the 21 records, 7 records were further excluded due to the sparse number of PTCL patients (number of PTCL patients < 10, n = 5), study design (retrospective study, n = 1), and a trial with duplicate population (n = 1).
The screening process is shown as a PRISMA flow chart (Fig 1). Using a modified 18-item checklist to evaluate study quality, all 14 studies that satisfied a minimum of 10 or more checklist items were considered high quality, with the number of items satisfied by each study ranging from 10 to 17. The detail records of quality assessment are shown in the supplementary materials (Table C in S1 File).
Fig 1. PRISMA 2009 Flow Diagram.
Study characteristics and Primary outcome
The basic characteristics of the selected studies are shown in Table 1. A total of 882 patients with NHL were initially identified from the 14 studies, and 618 of them were pertaining to relapsed/refractory PTCL. The most common subtypes were PTCL-NOS (n = 303, 49.0%), ALCL (n = 128, 20.7%), and AITL (n = 123, 19.9%). The patient numbers of relapsed or refractory PTCL ranged from 14 to 130. A total of 12 regimens were investigated, including 4 US FDA approved novel single-agent regimens (pralatrexate [13], romidespsin [14], brentuximab vedotin [15,20], belinostat [16]), 5 promising single-agent regimens (alemtuzumab [21], bendamustine [22], gemcitabine [23,24], lenalidomide [25], zanolimumab [26]), and 3 combination chemotherapies (13-cRA+interferon-α [27], A-DHAP [28], ICE [29]). The median age of study population from the included studies ranged from 46 to 66 years, and the median number of prior systematic therapies ranged from 1 to 3. Five of the 14 studies reported that some patients were previously treated with auto-HSCT, with the percentages ranging from 9% to 26%. Regarding clinical response assessments, only 4 of the 14 studies conducted independent or central reviews that indicated more rigorous assessment criteria than only local investigator (Table 1).
Table 1. Summary of characteristics of selected studies for patients with relapsed or refractory peripheral T-cell lymphoma treated with various salvage therapies.
| Study (year) | Regimen | Mechanism | No. of Patients, All/RR-PTCL | Age (year), median (range) | No. of prior therapy, median (range) | Prior auto-HSCT (%) | Independent Central Review |
|---|---|---|---|---|---|---|---|
| Enblad et al. (2004) | Alemtuzumab | Anti-CD52 monoclonal antibody | 14/14 | 61 (53–79) | 2 (1–4) | NR | No |
| Foss et al. (2015) | Belinostat | HDAC inhibitor | 53/24 | 64 (22.8–76.3) | N.R. | 5 (20.8%) | No |
| Damaj et al. (2013) | Bendamustine | Alkylating agent | 60/58 | 66 (43–87) | 1 (1–3) | NR | No |
| Pro et al. (2012) | Brentuximab vedotin | Anti-body drug conjugate | 58/58 | 52 (14–76) | 2 (1–6) | 15 (26%) | Yes |
| Horwitz et al. (2014) | Brentuximab vedotin | Anti-body drug conjugate | 35/35 | 64 (33–83) | 2 (1–9) | 3 (9%) | No |
| Zinzani et al. (2000) | Gemcitabine | Nucleoside analog | 44/14 | 58 (25–82)* | 3 (2–5)* | NR | No |
| Zinzani et al. (2010) | Gemcitabine | Nucleoside analog | 39/20 | 54 (32–78)* | 3 (2–8)* | NR | No |
| Morschhauser et al. (2013) | Lenalidomide | Immunomodulatory | 54/51 | 64.5 (39–86)* | 3 (1–11)* | NR | No |
| O'Connor et al. (2011) | Pralatrexate | Antifolates | 111/109 | 57.5 (21–85) | 3 (1–13) | 18 (16%) | Yes |
| Coiffier et al. (2012) | Romidepsin | HDAC inhibitor | 130/130 | 61 (20–83) | 2 (1–8) | 21 (16%) | Yes |
| d’Amore et al. (2010) | Zanolimumab | Anti-CD4 monoclonal antibody | 21/21 | 69 (26–85) | 2 (1–5) | NR | No |
| Huang et al. (2002) | 13-cRA+interferon-α | Combination therapy | 17/17 | 47 (18–77) | 1 (1–3) | NR | Yes |
| Seok et al. (2012) | A-DHAP | Combination therapy | 24/24 | 49 (23–60) | NR | NR | No |
| Zelenetz et al. (2003) | ICE | Combination therapy | 222/43 | 46 (NR)* | NR | No | No |
*indicates the statistics including data for some subtype other than PTCL
Abbreviations: A-DHAP: alemtuzumab, dexamethasone, cytarabine, cisplatin; ICE: ifosfamide, carboplatin, etoposide; HDAC: histone deacetylase; PTCL: peripheral T-cell Lymphoma; RR: relapsed or refractory; PTCL-NOS: peripheral T-cell Lymphoma, not otherwise specified; ALCL: anaplastic large cell lymphoma, ALCL(+): anaplastic lymphoma kinase positive ALCL, ALCL(-):anaplastic lymphoma kinase negative ALCL; AITL: angioimmunoblastic T-cell lymphoma; ENKTCL: extra-nodal NK/T cell lymphoma, nasal type; auto-HSCT: autologous hematopoietic stem cell transplantation; ORR: overall response rate; NR: not reported.
Primary outcome: overall response rate
The efficacy outcomes of relapsed/refractory PTCL treated with salvage therapy are shown in Table 2. Regarding primary efficacy outcome, the ORRs of included studies ranged from 22% treated with lenalidomide to 86% with brentuximab vedotin. Divided by the most frequently presented subtypes, the ORRs ranged from 14.2% to 71.5% for patients with the PTCL-NOS subtype, 8% to 54% for AITL subtypes, and 24% to 86% for the ALCL subtype, respectively (Table 2).
Table 2. Summary of characteristics of selected studies for primary and secondary outcomes in patients with relapsed or refractory peripheral T-cell lymphoma treated with various salvage therapies.
| Primary outcome: ORR (%) (95% CI) | Secondary outcomes, median (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|
| Study (year) | PTCL subgroup | All PTCL subtypes | PTCL-NOS | AITL | ALCL | DOR (month) | PFS (month) | OS (month) |
| Enblad et al. (2004) | PTCL-NOS 10 | 36 | 50 | NR | NR | 2.5 (1–8) | NR | NR |
| Foss et al. (2015) | PTCL-NOS 13, ALCL 3, AITL 3 | 25 (7.7–39.1) | NR | NR | NR | 3.63 (0.23–15.3) | 2.73 (1.2-NE) | NR |
| Damaj et al. (2013) | AITL 32, PTCL-NOS 23, ALCL 2 | 50 | NR | NR | NR | 3.5 (1–20.7) | 3.63 (2.41–5.19) | 6.27 (5.12–9.59) |
| Pro et al. (2012) | ALCL(+) 16, ALCL(-) 42 | 86 (74.6–93.9) | NR | NR | All: 86 (74.6–93.9), ALCL(-): 88, ALCL(+): 81 | 12.6 (5.7-NE) | 13.3 (6.9-NE) | NE (14.6-NE) |
| Horwitz et al. (2014) | PTCL-NOS 22, AITL 13 | 41 (24.6–59.3) | 33 (14.6–57) | 54 (25.1–80.8) | NR | 7.6 (1.3–14+) | 2.6 | NR |
| Zinzani et al. (2000) | PTCL-NOS 14 | 71.5 | 71.5 | NR | NR | CR: 15 (6–22), PR: 10 (2–15) | NR | NR |
| Zinzani et al. (2010) | PTCL-NOS 20 | 55 | 55 | NR | NR | CR: 34 (15–120) | NR | NR |
| Morschhauser et al. (2013) | AITL 26, PTCL-NOS 20 | 22* | 20 | 31 | NR | AITL, CR: 3.6 (3.5-NE) | All: 2.5 (1.8–4.6), AITL: 4.6 (1.8–8.2), non-AITL: 1.9 (1.6–3.3) | NR |
| O'Connor et al. (2011) | PTCL-NOS 59, ALCL 17, AITL 13 | 29* | 32 (21–46) | 8 (0–36) | 35 (14–62) | 10.1 (3.4-NE) | 3.5 (1.7–4.8) | 14.5 (1–24.1) |
| Coiffier et al. (2012) | PTCL-NOS 69, AITL 27, ALCL(-) 21 | 25 | 29 | 30 | ALCL(-): 24 | 16.6 (<0.1–34) | 4 | NR |
| d’Amore et al. (2010) | AITL 9, PTCL-NOS 7, ALCL 4 | 24 (8, 47) | 14.3 | 33.3 | 25.0 | NR | NR | NR |
| Huang et al. (2002) | PTCL-NOS 7, Ki-1 ALCL 6 | 31.3 (5.7–56.8) | 14.2 | NR | Ki-1 ALCL: 66.6 | 2.5 | 2.7 | 3.6 |
| Seok et al. (2012) | PTCL-NOS 13, ENKTCL 8 | 50 | 69.2 | NR | NR | 2.93 (0.93–4.93) | NR | 6 (4.2–7.8) |
| Zelenetz et al. (2003) | PTCL-NOS 26, ALCL 17 | 62.7 | 54 | NR | 77 | NR | NR | NR |
*indicates the statistics including data for some subtype other than PTCL
Abbreviations: A-DHAP: alemtuzumab, dexamethasone, cytarabine, cisplatin; ICE: ifosfamide, carboplatin, etoposide; HDAC: histone deacetylase; PTCL: peripheral T-cell Lymphoma; RR: relapsed or refractory; PTCL-NOS: peripheral T-cell Lymphoma, not otherwise specified; ALCL: anaplastic large cell lymphoma, ALCL(+): anaplastic lymphoma kinase positive ALCL, ALCL(-):anaplastic lymphoma kinase negative ALCL; AITL: angioimmunoblastic T-cell lymphoma; ENKTCL: extra-nodal NK/T cell lymphoma, nasal type; auto-HSCT: autologous hematopoietic stem cell transplantation; ORR: overall response rate; CR: complete response: PR: partial response; NR: not reported; NE: non-estimable DOR: duration of response; PFS: progression-free survival; OS: overall survival.
Secondary outcomes: duration of response, progression-free survival, overall survival
Nine of the 14 included studies reported DOR, and the medians ranged from 2.5 to 16.6 months. Seven of the 14 included studies reported PFS, and the medians ranged from 2.6 to 13.3 months. Only 5 of the 14 included studies reported OS, and the medians ranged from 3.6 to 14.5 months (Table 2).
Safety outcome: WHO grade 3 or 4 adverse events
The most frequent WHO grade 3 or 4 AEs for patients with relapsed or refractory PTCL treated by salvage therapy are summarized in Table 3. In general, grade 3 or 4 AEs were more likely hematological AEs for patients with relapsed or refractory PTCL, such as neutropenia (14% to 56%), thrombocytopenia (12% to 38%), leukopenia (28.5% to 78.2%), and anemia (11% to 18%). Those treated with gemcitabine [23, 24] did not suffer from grade 3 or 4 hematological AEs. The most frequently reported non-hematological AEs were infection (15% to 23%). Moreover, those treated with brentuximab vedotin reported grade 3 or 4 peripheral sensory neuropathy (9% to 12%) [15, 20], while those treated with pralatrexate showed grade 3 or 4 mucositis (22%) [13].
Table 3. Summary of most frequent adverse events with WHO grade 3 or 4 for patients with relapsed or refractory peripheral T-cell lymphoma treated by various salvage therapy.
| Study (year) | Regimen | No. of Patients treated | Hematological AE (%) | Non-hematological AE (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| leukopenia | neutropenia: | thrombocytopenia: | lymphopenia: | anemia: | hyperkalemia: | |||||
| Enblad et al. (2004) | Alemtuzumab | 14 | 28.5 | 21.4 | 14.2 | NR | ||||
| Foss et al. (2015) | Belinostat | 24 | 62.5 | NR | ||||||
| Damaj et al. (2013) | Bendamustine | 60 | 56 | 38 | infection: 20 | |||||
| Pro et al. (2012) | Brentuximab vedotin | 58 | 21 | 14 | peripheral sensory neuropathy: 12 | |||||
| Horwitz et al. (2014) | Brentuximab vedotin | 35 | 14 | 9 | peripheral sensory neuropathy: 9 | |||||
| Zinzani et al. (2000) | Gemcitabine | 44* | No grade 3 and grade 4 hematologic toxicity | NR | ||||||
| Zinzani et al. (2010) | Gemcitabine | 39* | No grade 3 and grade 4 hematologic toxicity | NR | ||||||
| Morschhauser et al. (2013) | Lenalidomide | 54 | 15 | 20 | gastrointestinal disorder: 17, infection: 15 | |||||
| O'Connor et al. (2011) | Pralatrexate | 111* | 15 | 32 | 18 | mucositis: 22 | ||||
| Coiffier et al. (2012) | Romidepsin | 131 | 20 | 24 | 11 | infection: 19 | ||||
| d’Amore et al. (2010) | Zanolimumab | 21 | NR | NR | ||||||
| Huang et al. (2002) | 13-cRA+interferon-α | 17 | 12 | 12 | infection: 23.5, fever: 12, fatigue: 12 | |||||
| Seok et al. (2012) | A-DHAP | 24 | 79.2 | 16.7 | NR | |||||
| Zelenetz et al. (2003) | ICE | 222* | NR | NR | ||||||
*indicates the statistics including data for some subtype other than PTCL
Abbreviations: A-DHAP: alemtuzumab, AEs, adverse events; dexamethasone, cytarabine, cisplatin; ICE: ifosfamide, carboplatin, etoposide; HDAC: histone deacetylase; PTCL: peripheral T-cell Lymphoma; RR: relapsed or refractory.
Cross-evaluation by both relative efficacy and safety
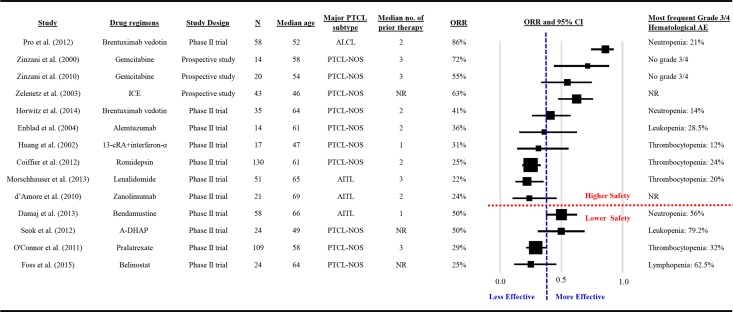
An ideal therapy regimen should be both effective and well-tolerated. The summary plot of ORR of the 14 studies is shown in Fig 2. The vertical, blue, dashed line indicates the threshold of relative efficacy (based on median of ORRs across studies, ORR > 38%), and the horizontal, red, dashed line indicates the cutoff of relative safety (most frequent grade 3 or 4 hematological AEs < 30%). If only phase II clinical trials were considered, those treated by brentuximab vedotin seemed to show both relatively higher efficacy and relatively higher safety (Fig 2).
Fig 2. Summary ORR plots of studies.
Discussion
Summary findings in this systematic review
In this study, we conducted a systematic review regarding salvage treatments on patients with relapsed or refractory PTCL. Systematic reviews can provide convincing and reliable evidence relevant to many aspects of medicine and health care; however, the conclusions are less clear when the included studies have differing results [30]. Through 2 levels of screening for eligible studies, a total of 14 studies covering 12 regimens were systematically reviewed. An apparent variation was observed in the ORRs across studies and regimens, along with various sample sizes, diverse distribution of PTCL subtypes, and different profiles of prior therapy. In this review, patients with the ALCL subtype responded to the salvage treatment (ORR: 24% to 86%), followed by those with PTCL-NOS (ORR: 14.2% to 71.5) and AITL (ORR: 8% to 54%). Among all 12 regimens, brentuximab vedotin had impressive ORR among those with the ALCL subtype (ORR: 86%). For the AITL subtype (ORR: 54%), romidepsin showed longer median duration of response (16.6 months) and pralatrexate showed longer median survival (14.5 months).
To the best of our knowledge, this is the first systematic review concerning salvage therapy regimens on relapsed/refractory PTCL. Our narrative review with comprehensive therapeutic options for relapsed or refractory PTCL, including different novel agents and stem cell transplantation, pointed out the development of new and effective treatment strategies for improving overall outcomes in patients with PTCL [5]. Moreover, a systematic review and meta-analysis was conducted for front-line anthracycline-based chemotherapy for PTCL, and it suggested that there is marked heterogeneity across PTCL subtypes in the benefits of anthracycline-based chemotherapy [8]. Compared to common aggressive B-cell lymphomas, patients with PTCL were more likely refractory to initial therapy and those with clinical responses tended to have shorter progression-free survival [1]. This indicates that novel agents and regimens are needed to improve outcomes for these patients, especially for the relapsed/refractory settings.
Heterogeneity in treatment efficacy across different salvage regimens
In 1994, the REAL Classification system for NHL explicitly distinguished B-cell and T-cell lymphomas with new subtypes based upon contemporary morphological, immunological, and genetic techniques [31]. The treatment paradigms for aggressive B-cell lymphoma were not effective for T-cell lymphoma [7]. Some salvage treatments previously stated to be effective for general relapsed/refractory lymphomas, such as DHAP [32] and ESHAP [33], were not as effective when administrated in relapsed/refractory PTCL specifically, as the clinical prognoses and treatment responses of B- and T-cell lymphomas are quite different. Even within PTCL, the prognoses and responses to therapy were varied across subtypes. An international study showed that the highest 5-year OS rates were observed in patients with primary cutaneous ALCL (90%) and ALK+ ALCL (70%), while poor 5-year OS rates were found for PTCL-NOS and AITL (both 32%) [6]. In this systematic review, the 14 included studies were highly heterogeneous in distribution of PTCL subtypes. Table 1 displays the numbers and profiles of prior therapy, demographic characteristics, review approaches for outcome assessment, and primary and secondary outcomes. These factors might be confounders for outcomes of different regimens. There is no head-to-head comparison among these studies. Some studies included heterogeneous subtype, but some recruited only a single subtype. There were two different treatment strategies: one single regimen constantly effective for different subtypes [13, 14], or subtype-specific regimens for optimal response [15]. Future studies are warranted to demonstrate which approach is more beneficial to relapsed/refractory PTCL.
Adverse events: well-tolerated or treatable?
One study did not report data of treatment toxicity [29], one did not show clear numbers of grade 3 or grade 4 adverse event [26], and two claimed no grade 3 or 4 hematologic toxicity [23, 24]. Ten studies had different frequencies of grade 3 or 4 hematologic AEs. An updated study on romidepsin demonstrated durable response with 28-month median DOR—the extended treatment and longer follow-up did not affect the reported safety profile of romidepsin. Regarding non-hematologic toxicity, 22% patients who received pralatrexate reported grade 3 or 4 mucositis. Generally, the adverse events caused by pralatrexate were manageable and most patients recovered and continued to receive the target dose of 30 mg/m2 pralatrexate for the duration of the therapy [12]. Leucovorin was demonstrated to reduce toxicity of pralatrexate without losing efficacy [34]. The adverse events were more severe if combination therapy was applied. The combination of single agents might result in improved outcomes; however, it also increases risk of treatment-related toxicity.
Therapeutic options
Outcomes of patients with PTCL are generally poor, with no well-established care standards for relapsed/refractory PTCL. Many options remain in the therapeutic abyss [7]. Treatment guidelines for PTCL [17,35] included stem cell transplantation as one therapeutic option. The fact that 5 of the 14 included studies included patients treated with auto-HSCT (9% to 20.8%) suggests that prior use of the therapy must have achieved enough of a response to warrant inclusion as a treatment option; however, the ultimate outcome of auto-HSCT in these 5 studies was neither durable nor efficacious. Allogeneic HSCT was demonstrated as a curative therapy for relapsed/refractory PTCL, provided that patients had suitable response to prior chemotherapy [36]. Studies showed the impressive efficacy of brentuximab vedotin in relapsed/refractory PTCL [15, 20]. The potential of immunotherapy was also promising for either front-line or second-line therapy for PTCL [35]. With many novel single agents demonstrating efficacy in relapsed/refractory PTCL, integrating these agents into conventional front-line chemotherapy is a major focus of study [12]. The phase III randomized trials comparing up-front multi-agent chemotherapy regimens and convention chemotherapy among previously untreated PTCL were reported ongoing [35].
Due to the rarity and heterogeneity of PTCL subtypes, the evidence for RCTs is very limited. By matched paired analysis [37,38], we could simulate randomized control data and compare patients treated with novel agents to those with conventional agents during a similar time period or with similar prognostic factors. So far, the largest cohort of PTCL consists of 1,314 cases from 22 centers worldwide [6]. The international, multicenter studies of large PTCL cohorts are valuable to establish a databank for long-term evaluations for patient outcomes and provide evidence-based practices [39].
Limitation
Our report presented a systematic review of best available data from existing publications on the salvage treatment of relapsed/refractory PTCL. Even though 4 electronic databases were searched, the studies regarding salvage treatment for relapsed/refractory PTCL were still very limited. Since PTCL is a relatively rare group of NHL, those with relapsed or refractory disease are even fewer. Moreover, lack of randomized control trials constrained the pool estimate of treatment effect. While the limited number of trials suggested an urgent need for relapsed/refractory PTCL treatment, it also decreased our likelihood for publication bias. Past studies have suggested that systematic reviews of randomized trials are the best strategy for appraising evidence; however, there have been instances where the findings of meta-analyses were later contradicted by large trials [40].
Conclusion
The efficacy of salvage therapy regimens is highly diverse for patients with relapsed/refractory PTCL, and the heterogeneity in the therapeutic effects might be due to the diversity in mechanisms, PTCL subtype distribution, numbers/profiles of prior therapy, and/or the assessment approaches. System review could provide convincing evidence relevant to many aspects of medicine; however, the conclusions are less clear when the included studies have differing effects, especially based on a limited number of small trials. The results of impressive efficacy and well-tolerated toxicity from these salvage therapy regimens should be treated with considerable caution. Use of comparative studies with matched pair analysis is warranted for more evidence on salvage treatment effects on relapsed or heavily pretreated patients with PTCL.
Supporting Information
(DOCX)
(DOC)
Acknowledgments
Disclaimer: The authors declare that the views expressed in the submitted article are his or her own and not an official position of the institution or funder.
Abbreviations
- A-DHAP
alemtuzumab, dexamethasone, cytarabine, cisplatin
- AEs
adverse event
- ALCL
anaplastic large cell lymphoma
- ALCL(+)
anaplastic lymphoma kinase positive ALCL
- ALCL(-)
anaplastic lymphoma kinase negative ALCL
- AITL
angioimmunoblastic T-cell lymphoma
- auto-HSCT
autologous hematopoietic stem cell transplantation
- CHOP
cyclophosphamide, doxorubicin, vincristine, and prednisone
- CI
confidence interval
- CTCL
cutaneous T-cell lymphoma
- DOR
duration of response
- ENKTCL
extra-nodal NK/T cell lymphoma, nasal type
- ESHAP
etoposide, methylprednisolone, cytarabine, cisplatin
- EPOCH
etoposide, prednisolone, vincristine, cyclophosphamide, doxorubicin
- FDA
Food and Drug Administration
- GDP
gemcitabine, dexamethasone, cisplatin
- GemOx
gemcitabine, oxaliplatin
- HDAC
histone deacetylase
- ICE
ifosfamide, carboplatin, etoposide
- MINE
mesna, ifosfamide, mitoxantrone, etoposide
- NCCN
National Comprehensive Cancer Network
- NHL
non-Hodgkin’s lymphomas
- PTCL
peripheral T-cell Lymphoma
- PTCL-NOS
peripheral T-cell Lymphoma, not otherwise specified
- ORR
overall response rate
- OS
overall survival
- PFS
progression-free survival
- NR
not reported
- RCTs
randomized controlled trials
- RR
relapsed or refractory
- SCT
stem cell transplantation
- WHO
World Health Organization
Data Availability
This is a systematic review and the secondary data were extracted from results of included papers.
Funding Statement
This study was supported by grants from “Golden Dream Project” launched by TaiShang Resources International Group in Taiwan. The funding body does not play roles in study design, interpretation of data and the manuscript drafting.
References
- 1.Lunning MA, Horwitz S. Treatment of peripheral T-cell lymphoma: are we data driven or driving the data? Curr Treat Options Oncol. 2013;14:212–223. 10.1007/s11864-013-0232-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vose JM. Peripheral T-cell non-Hodgkin’s lymphoma. Hematol Oncol Clin North Am. 2008;22:997–1005. 10.1016/j.hoc.2008.07.010 [DOI] [PubMed] [Google Scholar]
- 3.A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. The Non- Hodgkin’s Lymphoma Classification Project. Blood. 1997;89:3909–3918. [PubMed] [Google Scholar]
- 4.Karlin L, Coiffier B. The changing landscape of peripheral T-cell lymphoma in the era of novel therapies. Semin Hematol. 2014;51:25–34. 10.1053/j.seminhematol.2013.11.001 [DOI] [PubMed] [Google Scholar]
- 5.Coiffier B, Federico M, Caballero D, Dearden C, Morschhauser F, Jäger U, et al. Therapeutic options in relapsed or refractory peripheral T-cell lymphoma. Cancer Treat Rev. 2014;40:1080–1088. 10.1016/j.ctrv.2014.08.001 [DOI] [PubMed] [Google Scholar]
- 6.Vose J, Armitage J, Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: Pathology findings and clinical outcomes. J Clin Oncol. 2008;26:4124–4130. 10.1200/JCO.2008.16.4558 [DOI] [PubMed] [Google Scholar]
- 7.Foss F. Hematology: relapsed and refractory PTCL–into the therapeutic abyss. Nat Rev Clin Oncol. 2011;8:321–322. 10.1038/nrclinonc.2011.51 [DOI] [PubMed] [Google Scholar]
- 8.Abouyabis AN, Shenoy PJ, Sinha R, Flowers CR, Lechowicz MJ. A systematic review and meta-analysis of front-line anthracycline-based chemotherapy regimens for peripheral T-cell lymphoma. ISRN Hematol. 2011; 2011:623924 10.5402/2011/623924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mak V, Hamm J, Chhanabhai M, Shenkier T, Klasa R, Sehn LH, et al. Survival of patients with peripheral T-cell lymphoma after fırst relapse or progression: spectrum of disease and rare long-term survivors. J Clin Oncol. 2013;31:1970–1976. 10.1200/JCO.2012.44.7524 [DOI] [PubMed] [Google Scholar]
- 10.Morgensztern D, Walker GR, Koniaris LG, Lossos IS. Lack of survival improvement in patients with peripheral T-cell lymphoma: a Surveillance, Epidemiology, and End Results analysis. Leuk Lymphoma. 2011;52:194–204. 10.3109/10428194.2010.542596 [DOI] [PubMed] [Google Scholar]
- 11.Reddy NM, Evens AM. Chemotherapeutic advancements in peripheral T-cell lymphoma. Semin Hematol. 2014;51:17–24. 10.1053/j.seminhematol.2013.11.006 [DOI] [PubMed] [Google Scholar]
- 12.Cheah CY, Oki Y, Fanale MA. Novel treatments for T-cell lymphoma. Am Soc Clin Oncol Educ Book. 2015; 35:e468–478. 10.14694/edbook_am.2015.35.e468 [DOI] [PubMed] [Google Scholar]
- 13.O'Connor OA, Pro B, Pinter-Brown L, Bartlett N, Popplewell L, Coiffier B, et al. Pralatrexate in patients with relapsed or refractory peripheral T-cell lymphoma: results from the pivotal PROPEL study. J Clin Oncol. 2011;29:1182–1189. 10.1200/JCO.2010.29.9024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coiffier B, Pro B, Prince HM, Foss F, Sokol L, Greenwood M, et al. Results from a pivotal, open-label, phase II study of romidepsin in relapsed or refractory peripheral T-cell lymphoma after prior systemic therapy. J Clin Oncol. 2012;30:631–636. 10.1200/JCO.2011.37.4223 [DOI] [PubMed] [Google Scholar]
- 15.Pro B, Advani R, Brice P, Bartlett NL, Rosenblatt JD, Illidge T, et al. Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: results of a phase II study. J Clin Oncol. 2012; 30:2190–2196. 10.1200/JCO.2011.38.0402 [DOI] [PubMed] [Google Scholar]
- 16.Foss F, Advani R, Duvic M, Hymes KB, Intragumtornchai T, Lekhakula A, et al. A Phase II trial of Belinostat (PXD101) in patients with relapsed or refractory peripheral or cutaneous T-cell lymphoma. Br J Haematol. 2015;168:811–819. 10.1111/bjh.13222 [DOI] [PubMed] [Google Scholar]
- 17.National Comprehensive Cancer Network: NCCN clinical practice guideline on oncology: Non-Hodgkin’s lymphomas, Version 5, 2014. Available: http://www.nccn.org/professionals/physician_gls/pdf/nhl.pdf.Accessed 10 Dec 2014.
- 18.Sterne JAC, Higgins JPT, Reeves BC on behalf of the development group for ACROBATNRSI. A Cochrane Risk Of Bias Assessment Tool: for Non-Randomized Studies of Interventions (ACROBATNRSI), Version 1.0.0, 24 September 2014. Available: http://www.riskofbias.info. Accessed 30 Jun 2015.
- 19.Moga C, Guo B, Schopflocher D, Harstall C. Development of a Quality Appraisal Tool for Case Series Studies Using a Modified Delphi Technique. Edmonton AB: Institute of Health Economics, 2012.
- 20.Horwitz SM, Advani RH, Bartlett NL, Jacobsen ED, Sharman JP, O’Connor OA, et al. Objective responses in relapsed T-cell lymphomas with single-agent brentuximab vedotin. Blood. 2014; 123:3095–3100. 10.1182/blood-2013-12-542142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Enblad G, Hagberg H, Erlanson M, Lundin J, MacDonald AP, Repp R, et al. A pilot study of alemtuzumab (anti-CD52 monoclonal antibody) therapy for patients with relapsed or chemotherapy-refractory peripheral T-cell lymphomas. Blood. 2004; 103:2920–2924. 10.1182/blood-2003-10-3389 [DOI] [PubMed] [Google Scholar]
- 22.Damaj G, Gressin R, Bouabdallah K, Cartron G, Choufi B, Gyan E, et al. Results from a prospective, openlabel, phase II trial of bendamustine in refractory or relapsed T-cell lymphomas: the BENTLY trial. J Clin Oncol. 2013;31:104–110. 10.1200/JCO.2012.43.7285 [DOI] [PubMed] [Google Scholar]
- 23.Zinzani PL, Baliva G, Magagnoli M, Bendandi M, Modugno G, Gherlinzoni F, et al. Gemcitabine treatment in pretreated cutaneous T-cell lymphoma: experience in 44 patients. J Clin Oncol. 2000;18: 2603–2606. [DOI] [PubMed] [Google Scholar]
- 24.Zinzani PL, Venturini F, Stefoni V, Fina M, Pellegrini C, Derenzini E, et al. Gemcitabine as single agent in pretreated T-cell lymphoma patients: evaluation of the long-term outcome. Ann Oncol. 2010; 21:860–863. 10.1093/annonc/mdp508 [DOI] [PubMed] [Google Scholar]
- 25.Morschhauser F, Fitoussi O, Haioun C, Thieblemont C, Quach H, Delarue R, et al. A phase 2, multicentre, single-arm, open-label study to evaluate the safety and efficacy of single-agent lenalidomide (Revlimid) in subjects with relapsed or refractory peripheral T-cell non-Hodgkin lymphoma: the EXPECT trial. Eur J Cancer. 2013; 49:2869–2876. 10.1016/j.ejca.2013.04.029 [DOI] [PubMed] [Google Scholar]
- 26.d'Amore F, Radford J, Relander T, Jerkeman M, Tilly H, Österborg A, et al. Phase II trial of zanolimumab (HuMax-CD4) in relapsed or refractory non-cutaneous peripheral T cell lymphoma. Br J Haematol. 2010;150:565–573. 10.1111/j.1365-2141.2010.08298.x [DOI] [PubMed] [Google Scholar]
- 27.Huang CL, Lin ZZ, Su IJ, Chao TY, Tien HF, Chang MC, et al. Combination of 13-cis retinoic acid and interferon-alpha in the treatment of recurrent or refractory peripheral T-cell lymphoma. Leuk Lymphoma. 2002;43:1415–1420. 10.1080/1042819022386806 [DOI] [PubMed] [Google Scholar]
- 28.Kim SJ, Kim K, Park Y, Kim BS, Huh J, Ko YH, et al. Dose modification of alemtuzumab in combination with dexamethasone, cytarabine, and cisplatin in patients with relapsed or refractory peripheral T-cell lymphoma: analysis of efficacy and toxicity. Invest New Drugs. 2012;30:368–375. 10.1007/s10637-010-9523-2 [DOI] [PubMed] [Google Scholar]
- 29.Zelenetz AD, Hamlin P, Kewalramani T, Yahalom J, Nimer S, Moskowitz CH. Ifosfamide, carboplatin, etoposide (ICE)-based second-line chemotherapy for the management of relapsed and refractory aggressive non-Hodgkin’s lymphoma. Ann Oncol. 2003; 14(suppl 1):i5–i10. 10.1093/annonc/mdg702 [DOI] [PubMed] [Google Scholar]
- 30.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. British Medical Journal. 2003; 327:557 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harris NL, Jaffe ES, Stem H, Banks PM, Chan JK, Cleary ML, et al. A Revised European-American Classification of lymphoid neoplasms: A proposal from the International Lymphoma Study Group. Blood. 1994; 84:1361–1392. [PubMed] [Google Scholar]
- 32.Velasquez WS, Cabanillas F, Salvador P, McLaughlin P, Fridrik M, Tucker S, et al. Effective salvage therapy for lymphoma with cisplatin in combination with high dose Ara-C and dexamethasone (DHAP). Blood. 1998; 71:117–122. [PubMed] [Google Scholar]
- 33.Velasquez WS, McLaughlin P, Tucker S, Hagemeister FB, Swan F, Rodriguez MA, et al. ESHAP–an effective chemotherapy regimen in refractory and relapsing lymphoma: a 4-year follow-up study. J Clin Oncol. 1994;12:1169–1176. [DOI] [PubMed] [Google Scholar]
- 34.Koch E, Story SK, Geskin LJ. Preemptive leucovorin administration minimizes pralatrexate toxicity without sacrificing efficacy. Leuk Lymphoma. 2013;54:2448–2451. 10.3109/10428194.2013.779688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bachy E, Coiffier B. Time has come for immunotherapy in PTCL. Blood 2014; 123:3059–3060. 10.1182/blood-2014-04-565267 [DOI] [PubMed] [Google Scholar]
- 36.Goldberg JD, Chou JF, Horwitz S, Teruya-Feldstein J, Barker JN, Boulad F, et al. Long-term survival in patients with peripheral T-cell non- Hodgkin lymphomas after allogeneic hematopoietic stem cell transplant. Leuk Lymphoma. 2012;53:1124–1129. 10.3109/10428194.2011.645818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rades D, Huttenlocher S, Dunst J, Bajrovic A, Karstens JH, Rudat V, et al. Matched pair analysis comparing surgery followed by radiotherapy and radiotherapy alone for metastatic spinal cord compression. J Clin Oncol. 2010;28:3597–3604. 10.1200/JCO.2010.28.5635 [DOI] [PubMed] [Google Scholar]
- 38.Foerster R, Foerster FG, Wulff V, Schubotz B, Baaske D, Wolfgarten M, et al. : Matched-pair analysis of patients with female and male breast cancer: a comparative analysis. BMC Cancer. 2011;11:335 10.1186/1471-2407-11-335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raghupathi W, Raghupathi V. Big data analytics in healthcare: promise and potential. Health Inf Sci Syst. 2014;2:3 10.1186/2047-2501-2-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Egger M, Smith D, Minder C. Bias in meta-analysis detected by a simple, graphical test. British Medical Journal. 1997; 315:629 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOC)
Data Availability Statement
This is a systematic review and the secondary data were extracted from results of included papers.