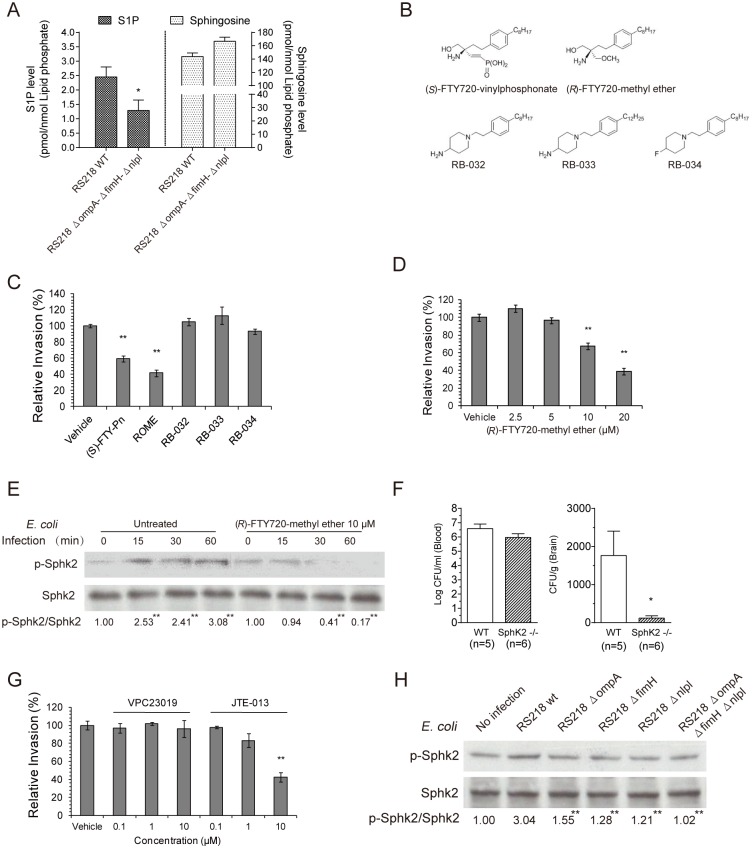
Fig 3. SphK2-S1P-S1P2 mediates meningitic E. coli penetration of the BBB in vitro and in vivo.
(A) S1P generation was significantly higher in HBMEC incubated with wild-type RS218 compared with the triple mutant deleted of ompA, fimH and nlpI. Correspondingly, the sphingosine level was lower in HBMEC incubated with wild-type RS218 than those incubated with the triple deletion mutant. * p<0.05. (B) Structures of (S)-FTY720-vinylphosphonae (SphK1 and SphK2 inhibitor), (R)-FTY720-methyl ether (selective SphK2 inhibitor), RB-032 and RB-033 (selective SphK1 inhibitors), and RB-034 (inactive analogue). (C) Both (S)-FTY720-vinylphosphonae (SphK1 and SphK2 inhibitor, shown as (S)-FTY-Pn) and (R)-FTY720-methyl ether (SphK2 inhibitor, shown as ROME) significantly inhibited RS218 invasion of HBMEC, while the SphK1 inhibitors (RB-032 and RB-033) and inactive analogue (RB-034) did not exhibit any inhibition. ** p<0.01. The inhibitors were all used at 10 μM. (D) (R)-FTY720-methyl ether inhibited E. coli RS218 invasion of HBMEC in a dose-dependent manner. ** p<0.01. (E) E. coli RS218 activated SphK2 in a time-dependent manner in HBMEC, while such activation was abolished by pretreatment with 10 μM (R)-FTY720-methyl ether. ** p<0.01. (F) E. coli penetration into the brain was significantly less in SphK2 −/− mice compared with wild-type mice. In contrast, the levels of bacteremia did not differ between the two groups of mice. (G) JTE-013 (S1P2 antagonist) significantly inhibited E. coli invasion of HBMEC, while VPC23019 (S1P1 and S1P3 antagonist) did not exhibit any inhibition. ** p<0.01. (H) The mutants with deletion of ompA, fimH, or nlpI as well as the triple mutant (ΔompAΔfimHΔnlpI) induced significantly lower levels of SphK2 activation in HBMEC, compared with wild-type RS218. ** p<0.01.