Figure 1.
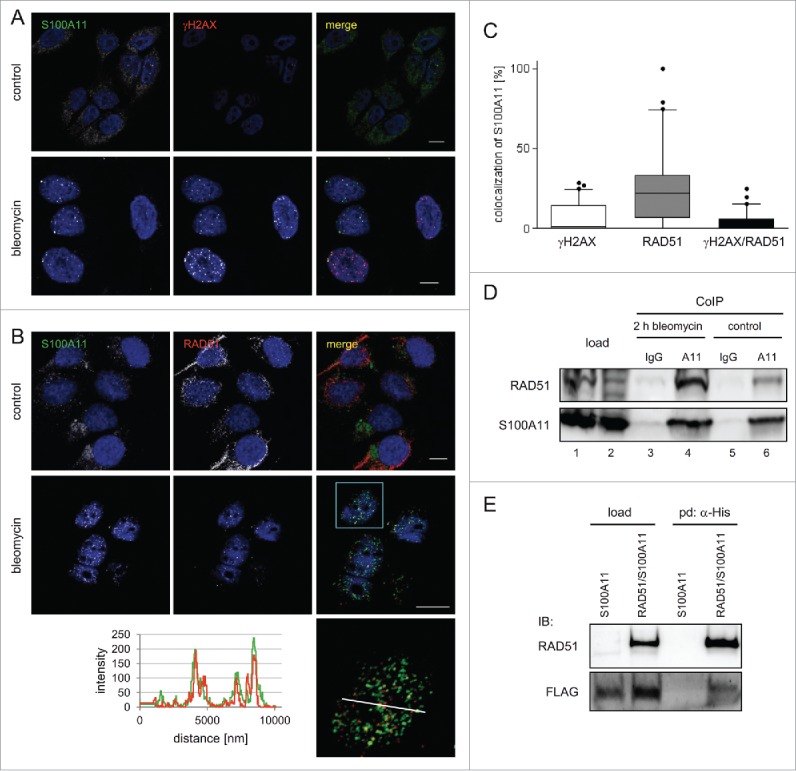
S100A11 interacts with RAD51 at sites of DSB repair. (A) HaCaT cells synchronized in S phase were treated with bleomycin (12.5 µg/ml) for 30 min and released in fresh medium for 90 min or were untreated (control) followed by immunostaining with antibodies against S100A11 (green) and γH2AX (red). Nuclear DNA was detected using DAPI (blue). Bar, 10 µm. (B) S phase HaCaT cells that were analyzed by laser scanning microscopy for S100A11 (green) and RAD51 (red) at sites of DNA damage 2 hour after bleomycin treatment. Bar, 10 µm. An area marked by a rectangle (light blue) in the overlay image (merge) is shown enlarged below the respective image. The intensities of the immunofluorescences in one cell derived from the RAD51 signal (red) and the S100A11 signal (green) are shown in a linescan (left side of the enlarged overlay). (C) Quantification of the colocalization of S100A11 with γH2AX (white Whisker box), RAD51 (gray Whisker box) and γH2AX/RAD51 (black Whisker box) in HaCaT cells after DSB induction. Colocalization events were determined in nuclei of cells treated as described in A. Thirty nuclei from 2 independent experiments were analyzed. Data are displayed as mean values (±SD). (D) Co-immunoprecipitation (CoIP) experiments between S100A11 and RAD51. A specific anti-S100A11 antibody precipitated RAD51 from whole cell extracts of S phase HaCaT cells treated with bleomycin (12.5 µg/ml) (lane 4) or untreated S phase cells (lane 6). In a control, an unspecific antibody used in CoIP experiments was unable to precipitate RAD51 from the same extracts (lanes 3 and 5). (E) The authenticity of the RAD51/S100A11 interaction was confirmed by pull-down with overexpressed proteins. U2OS cells were transfected with a plasmid encoding FLAG-S100A11 alone or together with a His-RAD51 encoding plasmid followed by synchronization in S phase and induction of DSBs by bleomycin treatment. Pull-down (pd) was carried out from cell extracts of the transfected cells for His-tagged proteins binding to Talon resins, and immunoblotting was performed using the antibodies as indicated. As loading control (load), 5% of the cell extract was used.
