Figure 4.
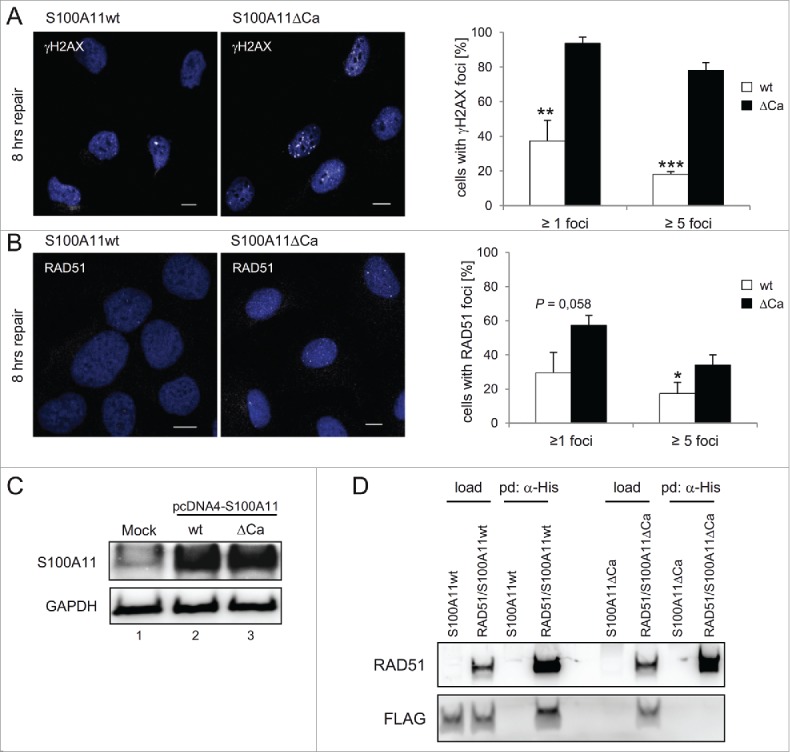
A S100A11 mutant without Ca2+-binding impairs DSB repair. (A) left panel Immunostaining of U2OS cells for γH2AX in cells expressing recombinant S100A11. Cells expressing S100A11ΔCa display significantly increased γH2AX levels 8 h after DSB induction. Cells transfected with a plasmid encoding S100A11wt or S100A11ΔCa, respectively, were synchronized in S phase, followed by treatment with 12.5 µg/ml bleomycin for 30 min. Cells were harvested 8 h after induction of DNA damage and analyzed by immunostaining against γH2AX (white). Nuclear DNA was detected using DAPI (blue). Bar, 10 µm. right panel Quantification of the percentage of cells showing γH2AX foci after S100A11wt (n = 88) or S100A11ΔCa (n = 99) transfection. The results of 3 independent experiments are presented. Data are shown as the mean ±SD. (B) Recombinant S100A11ΔCa expression increases RAD51 foci persistence. left panel U2OS cells were treated as above and analyzed by immunostaining against RAD51 (white). Nuclear DNA was detected using DAPI (blue). Bar, 10 µM. right panel Quantification of percentage of individual cells showing RAD51 foci. Mean values represent analysis of cells expressing S100A11wt (n = 94) or S100A11ΔCa (n = 99) of 3 independent experiments. (C) Expression of S100A11wt (lane 2) or S100A11ΔCa (lane 3) in pcDNA4-transfected U2OS cells was confirmed by immunoblotting against S100A11. An empty plasmid was used for mock-transfection of U2OS cells (lane 1). GAPDH served as loading control. (D) S100A11ΔCa mutant failed to interact with RAD51. U2OS cells were transfected with a plasmid encoding FLAG-S100A11 (wild-type or ΔCa mutant, respectively) alone or together with a His-RAD51 encoding plasmid followed by synchronization in S phase and induction of DSBs by bleomycin treatment. Pull-down (pd) was carried out from cell extracts of the transfected cells using Talon resins to precipitate His-tagged proteins together with interacting partners. For analysis of the pulled-down interacting proteins, immunoblotting was performed using the antibodies as indicated. As loading control (load), 5% of the cell extract was used. * P < 0.05, ** P < 0.01, *** P < 0.001.
