Figure 1.
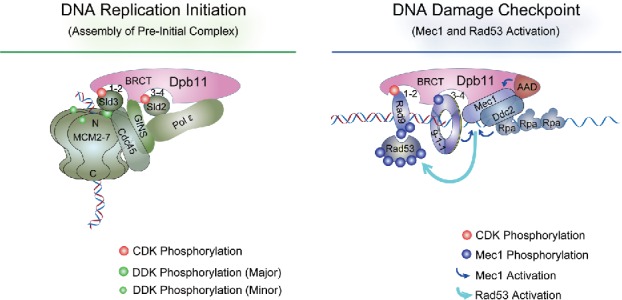
The roles of Dpb11 in DNA replication initiation and DNA damage checkpoint. The four BRCT domains of Dpb11 are depicted as 2 tBRCT pairs (BRCT1-2 and 3–4). As described in the text, Dpb11 binds Sld2 and Sld3, both phosphorylated by S-CDK, during DNA replication initiation (left panel).13,14 Dpb11 also interacts with GINS and Pol epsilon. Sld3, in complex with Cdc45, recognizes and interacts with MCM that is phosphorylated by DDK (Dbf4-dependent kinase).16,17 MCM, Cdc45 and GINS form the CMG complex that catalyzes template strand unwinding during replication.129 In DNA damage checkpoint (right panel), Dpb11 binds 9-1-1 that is located at 5′ ss- and ds-DNA junction and phosphorylated by Mec1.24,26 Dpb11 also binds to CDK-phosphorylated Rad9, which can associate with chromatin by interacting with γH2A and H3 with K79 methylation (not depicted here).25,26 Subsequently Mec1 is activated by Ddc2, Ddc1 of the 9-1-1 complex, and the AAD (ATR-activation domain) of Dpb11.29,30,130 Mec1 then phosphorylates and activates Rad53, which is recruited to DNA lesion sites by its association with phosphorylated Rad9.31,32
