Figure 5.
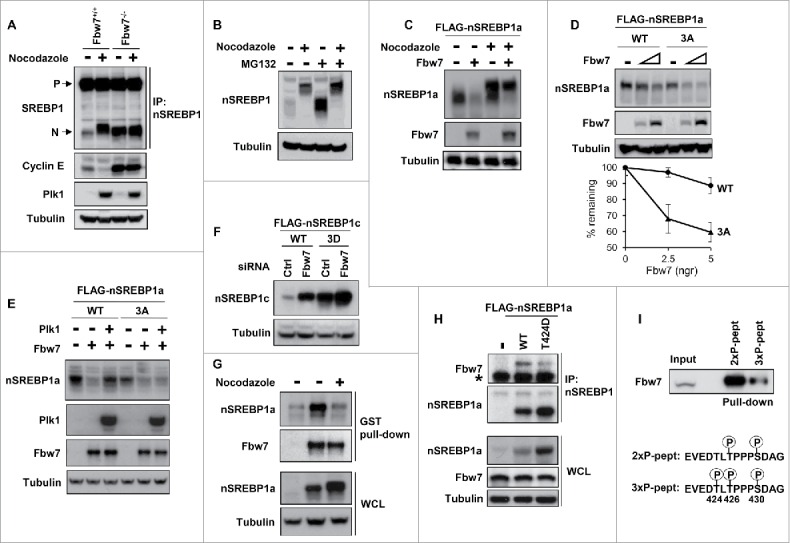
Plk1-mediated phosphorylation of nuclear SREBP1 prevents its degradation by Fbw7. (A) WT (Fbw+/+) or Fbw7 knockout (Fbw7−/−) HCT116 cells were treated in the absence or presence of nocodazole. The levels of SREBP1, Cyclin E, Plk1 and α-tubulin (loading control) were determined by Western blotting. The membrane-associated precursor (P) and nuclear (N) forms of SREBP1 are indicated. (B) HeLa cells were left untreated or treated with nocodazole to induce mitotic arrest. Where indicated, cells were treated with the proteasome inhibitor MG-132 (25 μM) for the last 4 h. The levels of nSREBP1 and α-tubulin were determined by Western blotting. (C) HEK293 cells were transfected with nSREBP1a in the absence or presence of Fbw7α and the cells were left untreated or treated with nocodazole. The levels of nSREBP1a, Fbw7α and α-tubulin were determined by Western blotting. (D) HEK293 cells were transfected with nSREBP1a, either WT or the 3A mutant, in the absence or presence of increasing amounts of Fbw7α. The levels of nSREBP1a, Fbw7α and α-tubulin were determined by Western blotting (upper panel). The percentage of nSREBP1a remaining is plotted against the concentration of Fbw7α (lower panel). The data represent the averages ± SD of three independent experiments. (E) HEK293 cells were transfected with nSREBP1a, either WT or the 3A mutant, in the absence or presence of Plk1 and Fbw7α. The levels of nSREBP1a, Plk1, Fbw7α and α-tubulin were monitored by Western blotting. (F) HEK293 cells were transfected with nSREBP1c, either WT or the 3D mutant, together with control or Fbw7 siRNA. The levels of nSREBP1c and α-tubulin were determined by Western blotting. (G) HEK293 cells were transfected with nSREBP1a and either left untreated or treated with nocodazole. Lysates from the transfected cells were mixed with recombinant GST-Fbw7α. Following capture of GST-Fbw7α and extensive washing, the bound proteins were subject to SDS/PAGE and the amount of nSREBP1a and Fbw7α were determined by Western blotting (upper panel). The amount of nSREBP1a and α-tubulin in whole cell lysates (WCL) were determined by Western blotting (lower panel). (H) HEK293 cells were transfected with FLAG-nSREBP1a, either WT or the T424D mutant. Cell lysates were immunoprecipitated with anti-FLAG antibodies. The amounts of immunoprecipitated Fbw7 and nSREBP1a (upper panel), and the levels of nSREBP1a, Fbw7 and α-tubulin in cell lysates (Input, lower panel) were determined by Western blotting. The band indicated by the asterisk corresponds to the IgG chain. (I) HeLa cell lysates were used in peptide pull-down assays, using 2 peptides corresponding to residues 422–442 of human SREBP1a, either phosphorylated on T426 and S430 (2xP-pept) or the same peptide phosphorylated on T424, T426 and S430 (3xP-pept). The bound proteins were subjected to SDS/PAGE and Western blotting using 20% of input as control.
