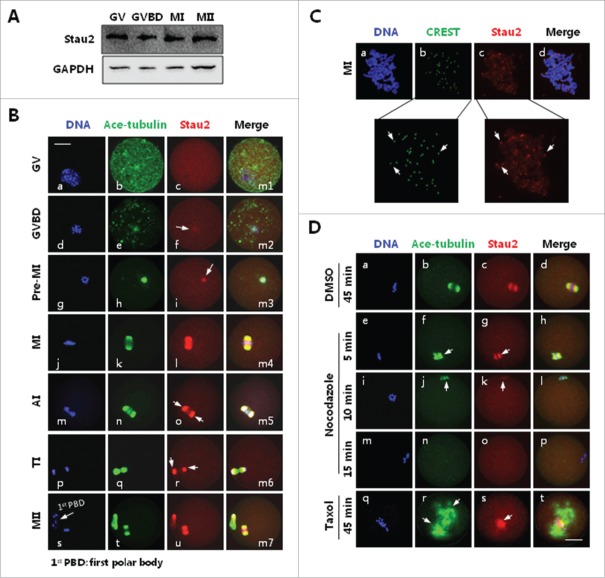
Figure 1.
Expression and subcellular localization Stau2 in mouse oocyte during meiosis. Mouse oocytes were cultured in vitro in MEM medium with 10 % FBS for 0, 2, 4, 8, 12,17 h, corresponding to meiotic stages of germinal vesicle (GV), germinal vesicle breakdown (GVBD), pro-metaphase I (pro-MI), metaphase I (MI), anaphase I/telophase I (AI/TI) and metaphase II (MII), respectively, and collected for analysis with western blot and immunofluorescence. A. Western blot analysis revealed stable expression of Stau2 in mouse oocytes during meiotic division. Protein samples of 50 oocytes, at GV, GVBD, MI and MII stages, respectively, were prepared and segregated on 10% SDS-PAGE separation gel and immunoblotted with Stau2, GAPGH was placed as a loading control. B. Immunofluorescence analysis detected dynamic subcellular distribution in oocytes during meiosis. No special accumulation of Stau2 was detected at GV stage (c). Upon GVBD, Stau2 was aggregated as filaments and colocalized with microtubules around condensing chromosomes (g: arrow). During the meiotic progression from pro-MI to MI, Stau2 was gradually organized into bipolar structure and precisely overlapped with the meiotic spindle assembled from microtubules (j, k, n, o). During AI to TI transition, Stau2 remained colocalized with microtubules but excluded in the midbody structure (r, s: arrows). As oocytes developed to MII stage, Stau2 was assembled into “spindle-like” organization again and overlapped with the MII spindle, Stau2 was also colocalized with microtubules in first polar body (u-x). Stau2 was visualized in red, microtubule was labeled with antibody against acetylated-tubulin (ace-tubulin) and visualized in green, and DNA was visualized in blue. Scale bar = 20 μm. C. Immunostaining analysis revealed Stau2 accumulation across chromosomes, with particular concentration on the centromere areas (arrows in boxes). Stau2 was visualized in red, centromere was probed with CREST auto serum and visualized in green, and DNA was visualized in blue. Scale bar = 20 μm. D. Stau2 distribution was associated with microtubule integrity and stability in oocytes. MI oocytes were treated with 20 μg/ml nocodazole for 5, 10 and 15 min or 10 μM taxol for 45 min, then fixed for immunostaining with acetylated-tubulin (ace-tubulin) and Stau2. During incubation with nocodazole, Stau2 was gradually disassembled (f, g, n) together with microtubules (g, k, o). After taxol treatment, microtubules were stabilized in spindle and cytoplasmic microtubule organizing centers (MTOCs) (r: arrows), meanwhile Stau2 was congressed in the vicinity around chromosome alignment and absent on microtubules (s, t). Microtubules were visualized in green, Stau2 was visualized in red and DNA was labeled in blue. Scale bar = 20 μm.