Figure 7. RSK-mediated regulation of PDCD4 is required for the proliferation, survival, and migration of MDA-MB-231 cells.
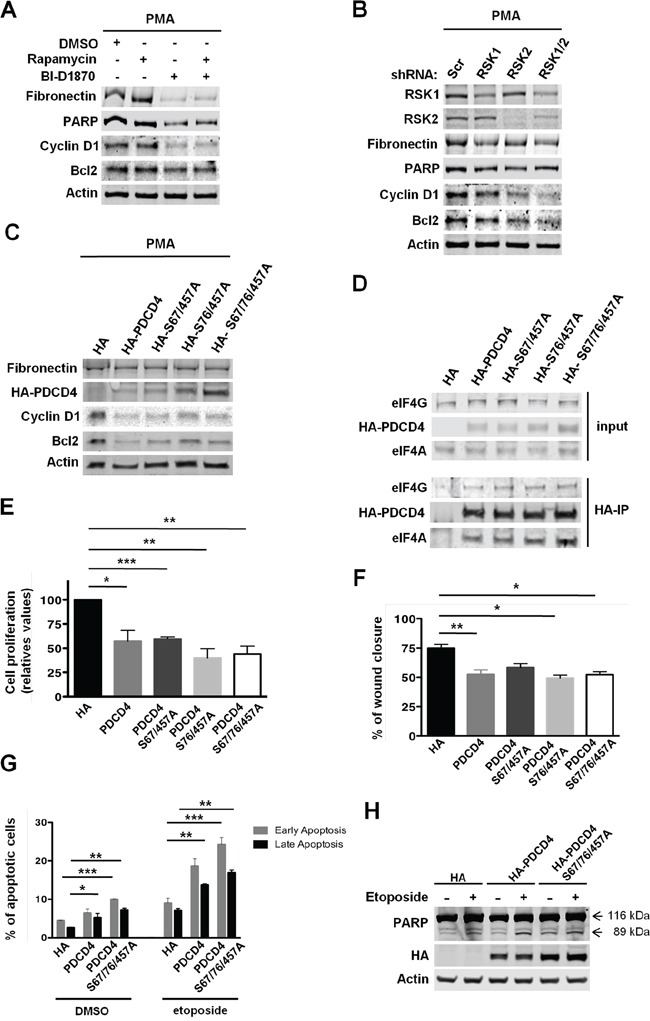
A. MDA-MB-231 cells were grown in serum-free media with PMA (50 ng/ml) and vehicle (DMSO), rapamycin (20 nM), and/or BI-D1870 (10 μM) for 24 h. Whole-cell extracts were obtained and resolved by SDS-PAGE. Indicated proteins were analyzed by immunoblotting with specific antibodies. B. MDA-MB-231 cells were infected with lentiviruses expressing shRNAs targeted against a scrambled sequence (Scr), RSK1, RSK2, or RSK1/2. After selection, cells were grown in serum-free media with PMA (50 ng/ml) for 24 h. Cell extracts were resolved by SDS-PAGE, and indicated proteins were analyzed by immunoblotting with specific antibodies. C. MDA-MB-231 cells transiently expressing HA tag, HA-tagged PDCD4, HA-tagged PDCD4 (S67/457A), HA-tagged PDCD4 (S76/457A), or HA-tagged PDCD4 (S67/76/457A) were selected and then grown in serum-free media with PMA (50 ng/ml) for 24 h. Indicated proteins were analyzed by immunoblotting with specific antibodies. D. Whole-cell extracts were obtained from the cells described in C. Equal amounts of total proteins were used to immunoprecipitate HA-tagged PDCD4 proteins using anti-HA agarose beads. Immunocomplexes and 1/10 of the protein used for immunoprecipitation (input) were resolved by SDS-PAGE, and indicated proteins were analyzed by immunoblotting with specific antibodies. E. MDA-MB-231 cells described in C were grown in 0.5% FBS media with PMA (50 ng/ml) for 3 days. Viable cells were estimated by neutral red uptake assays, and values represented as mean percentage ± SEM relative to HA tag-expressing cells (100%) determined from three independent assays (*p<0.05; **p<0.01; ***p< 0.001). F. Cells described in C were subjected to wound healing assays in 1% FBS media. Percentage of wound recovery was determined as described in Materials and Methods, and results were represented as means ± SEM. (*p<0.05; **p<0.01; ***p<0.001). G. Cells expressing HA tag, HA-tagged PDCD4, or HA-tagged PDCD4 (S67/76/457A) were selected and then treated with either vehicle (DMSO) or etoposide (50μM) for 24 hours. Early and late apoptotic cells were labeled with Guava Nexin Reagent, and quantified using the Guava EasyCyte Flow Cytometer. Percentage of apoptotic cells was represented as means ± SEM. (*p<0.05; **p<0.01; ***p<0.001). H. The levels of full-length (116 kDa) and cleaved (89 kDa) PARP, HA-PDCD4, and HA-PDCD4 (S67/76/457A) in cells treated as in G were determined by immunoblotting.
