Figure 5. Se-PFPs suppress β-catenin signaling in a GSK-3β–dependent mechanism.
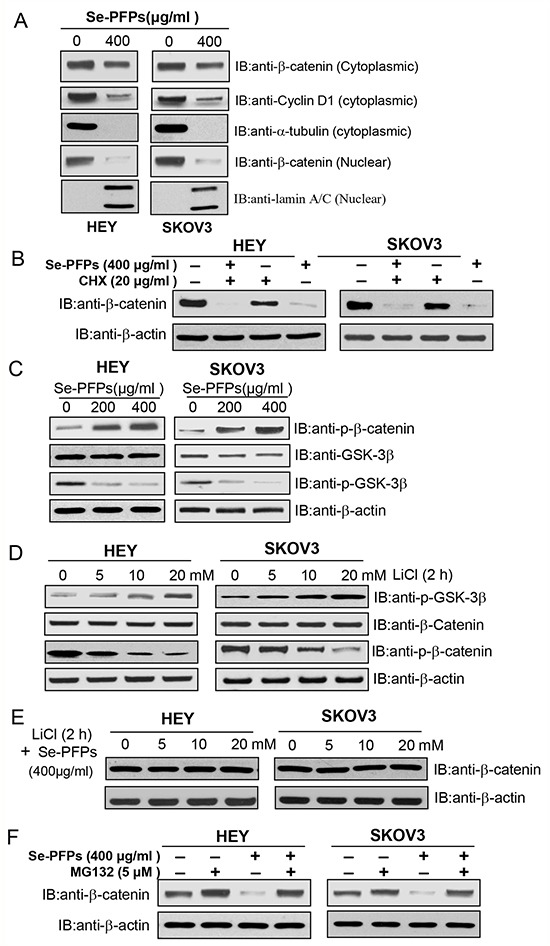
A. Se-PFPs inhibit the expression of cytoplasmic and nuclear β-catenin and cyclin D1 in HEY and SKOV3 cells. HEY and SKOV3 cells were incubated with the indicated doses of Se-PFPs for 48 hrs. α-tubulin was used as a loading control for cytoplasmic proteins, while lamin A/C was an internal control for nuclear proteins. B. Protein synthesis inhibitor, cycloheximide (CHX), reduces β-catenin expression in Se-PFPs-treated HEY and SKOV3 cells. The cells were incubated with 20 μg/ml of CHX in the presence or absence of Se-PFPs (400 μg/ml) for 24 hrs. C. Se-PFPs inhibit GSK-3β phosphorylation, leading to elevated phosphorylation of β-catenin in HEY and SKOV3 cells. HEY and SKOV3 cells were incubated with the indicated doses of Se-PFPs for 48 hrs. D. GSK-3β inhibitor, LiCl, increases GSK-3β phosphorylation and thus inhibits β-catenin phosphorylation in HEY and SKOV3 cells. The cells were treated with indicated concentrations of LiCl for 2 hrs. E. LiCl completely neutralizes the inhibitory effect of Se-PFPs on β-catenin expression. HEY and SKOV3 cells were treated with different doses of LiCl for 2 hrs in the presence of 400 μg/ml Se-PFPs. F. Proteasome inhibitor MG132 can rescue β-catenin expression in Se-PFPs-treated HEY and SKOV3 cells. HEY and SKOV3 cells were treated with Se-PFPs for 18 hrs, and co-treated with 5 μM MG132 for another 6 hrs. β-actin was used as a loading control.
