Figure 6. FGFR2 extracellular or cytoplasmic domain deletion reduced aggregated colocalization while retaining diffuse colocalization with CD44.
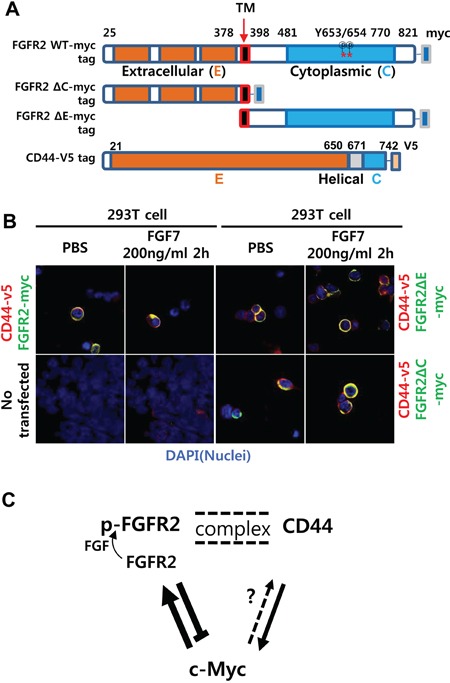
A. Schemes of myc-tagged FGFR2IIIc (FGFR2-myc) mutants with deleted N terminal extracellular (E) or C terminal cytoplasmic (C) domains. Note there are 29 phosphorylation sites in the wild type (WT) (18 threonine/serine, 11 tyrosine). Two phospho-tyrosine sites (a. a. residues 653 and 654) recognized by the antibody used in this study are indicated with asterisks (*). B. Without FGF7 treatment (PBS), the full-length FGFR2 WT colocalized with CD44 in a diffuse pattern on and/or near the cell surface. With FGF7 treatment, the two molecules aggregated to a single punctate dot per cell. FGFR2 ΔE and FGFR2 ΔC showed only diffuse staining with no evident aggregates regardless of FGF7 treatment. Untransfected 293T cells were used as a negative control to validate the absence of v5 and detectable endogenous myc. C. A proposed model of the reciprocal regulatory circuit. Arrows (→) and bars (┴) indicate activation and repression, respectively. Double dotted lines indicate FGF7-induced formation of punctate complexes between the two proteins. Less conclusive regulation is shown as a dotted arrow. p-FGFR2 is the phosphorylated form of FGFR2.
