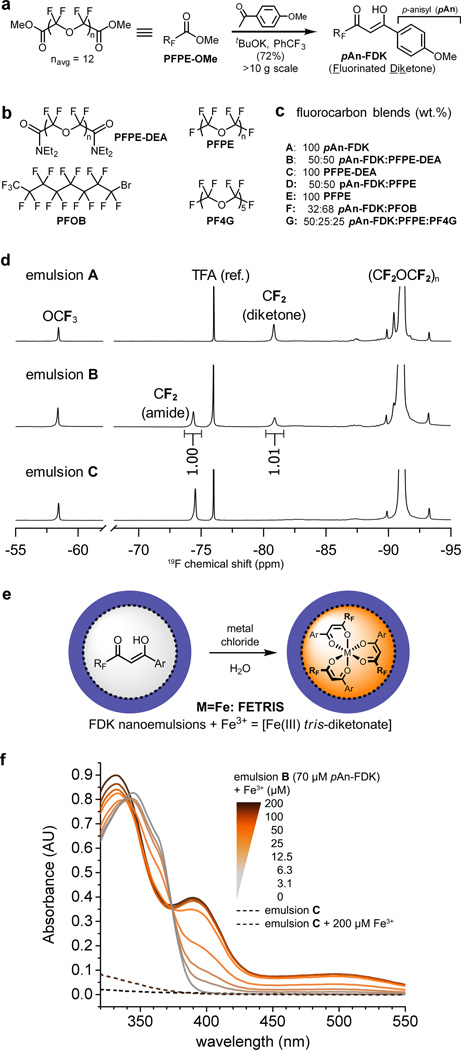
Figure 2. Preparation and characterization of metal-binding nanoemulsions for 19F MRI.
a, Synthesis of metal-binding fluorinated diketones (FDK) from PFPE-OMe (denoted as RFCO2Me). b, Structures of fluorocarbons used for 19F MRI. c, Composition and preparation of various metal-binding (A, B, D, F, G) and control (C, E) fluorocarbon nanoemulsions. d, 19F NMR spectra (11.7 T) of emulsions A–C (4.5 g/L 19F, 90% D2O). Signals from terminal CF2 of diketone ligands are well separated from other peaks and are used to determine ligand concentration. The peak at −76 ppm is the reference (CF3CO2Na, TFA). e, Addition of aqueous metal chlorides to FDK emulsions yields metalated emulsions; Ar = pAn. f, Absorption spectra of metal-binding emulsion B (70 µM diketone, 0.09 g/L 19F) (color) and control emulsion C (0.09 g/L) (---) in the presence of Fe3+. Increasing [Fe3+] causes the appearance of ferric tris-diketonate charge transfer bands at 395 and 500 nm that grow linearly in intensity until the ca. 3:1 ligand:Fe ratio is reached at 25 µM Fe3+.