Abstract
Background and objectives
The optimal timing of predialysis arteriovenous fistula surgery remains uncertain. We evaluated factors associated with hemodialysis initiation in patients undergoing predialysis arteriovenous fistula surgery and derived a model to predict future initiation of dialysis.
Design, setting, participants, & measurements
Our study retrospectively identified 308 patients undergoing predialysis arteriovenous fistula creation at a large medical center in 2006–2012 to determine whether they initiated hemodialysis. Multiple variable logistic regression analyzed which demographic and clinical factors predicted initiation of dialysis within 2 years of arteriovenous fistula surgery. A receiver operating characteristic area under the curve was used to quantify the predictive value of preoperative factors on the likelihood of initiating hemodialysis within 2 years.
Results
Overall, hemodialysis was initiated within 6 months, 1 year, and 2 years in 119 (39%), 175 (57%), and 211 (68%) patients, respectively. Using multiple variable logistic regression, four factors were associated with hemodialysis initiation at 2 years: eGFR at access surgery (odds ratio, 0.45; 95% confidence interval, 0.31 to 0.64 per 5 ml/min per 1.73 m2; P<0.001), diabetes (odds ratio, 2.51; 95% confidence interval, 1.22 to 5.15; P=0.003), GFR trajectory (odds ratio, 1.54; 95% confidence interval, 1.09 to 2.17 per 3 ml/min per 1.73 m2 per year; P=0.01), and spot urine protein-to-creatinine ratio (odds ratio, 1.39; 95% confidence interval, 1.14 to 1.71 per 1 U; P<0.001). eGFR alone had a moderate predictive value for dialysis initiation (area under the curve =0.69; 95% confidence interval, 0.63 to 0.76; P<0.001), whereas the full model had a higher predictive value (area under the curve =0.83; 95% confidence interval, 0.77 to 0.88; P<0.001).
Conclusions
The likelihood of initiating hemodialysis within 2 years of predialysis arteriovenous fistula surgery is associated with eGFR at access surgery, diabetes, GFR trajectory, and magnitude of proteinuria. The combined use of all four variables improves the ability to predict future hemodialysis compared with the use of eGFR alone.
Keywords: arteriovenous access, arteriovenous fistula, glomerular filtration rate, Area Under Curve, creatinine, diabetes mellitus, Humans, Logistic Models, Probability, proteinuria, ROC Curve, renal dialysis
Introduction
Nearly 80% of United States patients initiate hemodialysis with a central venous catheter rather than a permanent vascular access (1), placing these patients at an increased risk of infection, hospitalization, morbidity, and mortality (1–6). Substantial efforts have been undertaken by nephrologists to increase the use of permanent vascular access at hemodialysis initiation, specifically arteriovenous fistulas (AVFs). One major challenge to increasing the use of AVFs at hemodialysis initiation has been the lack of evidence regarding the optimal timing of preemptive vascular access placement (7). If the AVF is placed too late, the patient will likely start dialysis with a central venous catheter. However, if the AVF is placed too early, it may never be used, because the patient dies before requiring dialysis or does not require initiation of dialysis for a prolonged period. The current guidelines from the Fistula First Catheter Last Initiative (8) and the 2006 Kidney Disease Outcomes Initiative (9) recommend that “a fistula should be placed at least 6 months or with sufficient lead time before the anticipated start of hemodialysis treatments for fistula maturation” (8,9). The Fistula First Change Concept 3 recommends that nephrologists refer patients to surgeons for “AVF only” evaluation no later than stage 4 CKD (eGFR<30 ml/min per 1.73 m2) (8). This timing allows for vascular access placement and additional time for revision to ensure that a working AVF is available at initiation of dialysis therapy.
In comparison, the Canadian Society of Nephrology recommends placement of the AVF when eGFR is between 15 and 20 ml/min per 1.73 m2 (10), the Japanese Society for Dialysis Therapy recommends AVF placement when the creatinine clearance is between 10 and 20 ml/min or serum creatinine is between 6 and 8 mg/dl (11), and the European Best Practices Guidelines recommend vascular access placement when the eGFR reaches <30 ml/min per 1.73 m2 (12). Because these national guidelines are not on the basis of clinical evidence and because none of them incorporate clinical patient characteristics, the optimal timing of predialysis vascular access placement remains unknown. The primary objective of this study was to assess the predictive value of eGFR at surgery and other clinical factors that may be associated with initiation of hemodialysis in patients undergoing predialysis AVF surgery. A secondary objective was to determine which factors predicted use of a permanent vascular access at initiation of hemodialysis.
Materials and Methods
Vascular Access Management
The University of Alabama at Birmingham (UAB) provides ESRD care for approximately 600 patients on in-center hemodialysis at 10 units in the metropolitan Birmingham area. Ten full–time clinical nephrologists at the UAB provide care for these patients on hemodialysis. Four experienced UAB transplant surgeons perform all vascular access creations for our patients with CKD, and the Department of Radiology and Division of Nephrology performs all percutaneous interventional procedures for vascular access.
Preoperative vascular access mapping was performed as the standard of care to assist the surgeon in planning of AVF surgery (13–15). The minimal arterial diameter was 2 mm, and the minimal venous diameter was 2.5 mm. Six-week postoperative ultrasounds were performed as the standard of care to evaluate AVF maturation (16,17). AVFs deemed immature by postoperative ultrasound were referred to Transplant Surgery or Interventional Nephrology/Radiology for salvage procedures. AVFs were allowed to mature for 8–12 weeks before their initial cannulation.
Data Collection
Two full–time vascular access coordinators in the Division of Nephrology scheduled all vascular procedures and maintained a prospective vascular access database that was used to track all vascular access procedures and outcomes in our dialysis program (13). Institutional Review Board approval was obtained before review of the patients’ medical records for research purposes. We retrospectively queried our prospective vascular access database to identify all predialysis AVFs placed from January 1, 2006 to December 31, 2012. During this time period, 372 patients with CKD underwent predialysis AVF surgery. We excluded 64 patients (17%), because the serum creatinine was not available on the surgery date or in the preceding 1 month. The remaining 308 patients with known eGFR on the surgery date were the focus of this analysis. The 64 patients who were excluded because of missing eGFR at AVF surgery were similar to those included in the analysis with respect to age (57±13 versus 56±14 years old), women (48% versus 47%), diabetes (59% versus 58%), and other comorbidities. Each patient’s electronic medical record was reviewed to collect demographic and clinical information as well as vascular diameters obtained during the preoperative vascular ultrasound. The eGFR trajectory (decrease in eGFR) in the year preceding AVF surgery was estimated from the slope of all eGFR values available for that time period. Proteinuria was estimated from the ratio of protein to creatinine concentration in a spot urine collection (with both values expressed in milligrams per deciliter) as long as a value was available within the 6 months preceding AVF surgery. BP control was estimated using the first preoperative systolic BP obtained on the AVF surgery date. For the subset of patients who started dialysis, we ascertained whether they used a permanent vascular access on their first hemodialysis session and whether the access was still in use 1 month after initiating dialysis.
Statistical Analyses
Baseline patient characteristics were compared between eGFR groups using chi-squared tests for categorical variables. We used multiple variable logistic regression analysis to model the association between baseline variables and the likelihood of hemodialysis initiation within 2 years. Receiver operator characteristics (ROC) curves were used to assess the predictive value of baseline clinical factors at access surgery for the likelihood of initiating hemodialysis within 2 years. P values <0.05 were considered statistically significant.
Results
Patient Characteristics
The study cohort included 308 patients with CKD who underwent predialysis AVF placement at our medical center during a 7-year period for whom an eGFR was available on the surgery date. The patients were divided into four cohorts on the basis of their eGFR values at the time of surgery (Table 1). Only the frequency of coronary artery disease and magnitude of proteinuria differed significantly among the four cohorts. Age, sex, race, diabetes, hypertension, peripheral vascular disease, cerebrovascular disease, congestive heart failure, GFR trajectory, and systolic BP were similar in the four groups.
Table 1.
Baseline characteristics of patients who were pre-ESRD with arteriovenous fistulas by eGFR group
| Patient Characteristic | eGFR at Access Surgery, ml/min per 1.73 m2 | P Value | |||
|---|---|---|---|---|---|
| <10 | 10–14 | 15–19 | ≥20 | ||
| N patients | 30 | 115 | 101 | 62 | |
| Age ≥65 yr old | 9 (30%) | 35 (30%) | 36 (36%) | 17 (27%) | 0.71 |
| Women | 14 (47%) | 55 (48%) | 42 (42%) | 33 (53%) | 0.54 |
| Black | 22 (73%) | 78 (68%) | 72 (72%) | 40 (64%) | 0.76 |
| DM | 13 (43%) | 69 (60%) | 61 (60%) | 35 (56%) | 0.37 |
| HTN | 29 (97%) | 113 (98%) | 97 (96%) | 60 (97%) | 0.99 |
| CAD | 6 (10%) | 26 (23%) | 39 (39%) | 10 (16%) | <0.01 |
| PVD | 3 (7%) | 12 (10%) | 18 (18%) | 5 (8%) | 0.22 |
| CHF | 7 (23%) | 31 (27%) | 31 (31%) | 26 (42%) | 0.16 |
| GFR trajectory, ml/min per 1.73 m2 per yr, median [IQR] | 5 [4–7] | 5 [4–8] | 5 [4–8] | 4 [2–7] | 0.05 |
| Urine protein-to-creatinine ratio,a median [IQR] | 2.3 [0.8–3.3] | 2.0 [1.1–4.5] | 1.6 [0.5–3.2] | 0.9 [0.3–2.9] | 0.002 |
| Systolic BP, mmHg, mean±SD | 147±29 | 150±26 | 148±22 | 144±25 | 0.53 |
DM, diabetes; HTN, hypertension; CAD, coronary artery disease; PVD, peripheral vascular disease; CHF, congestive heart failure; IQR, interquartile range.
Both urine protein and urine creatinine are in milligrams per deciliter.
Likelihood of Hemodialysis Initiation after Predialysis AVF Placement
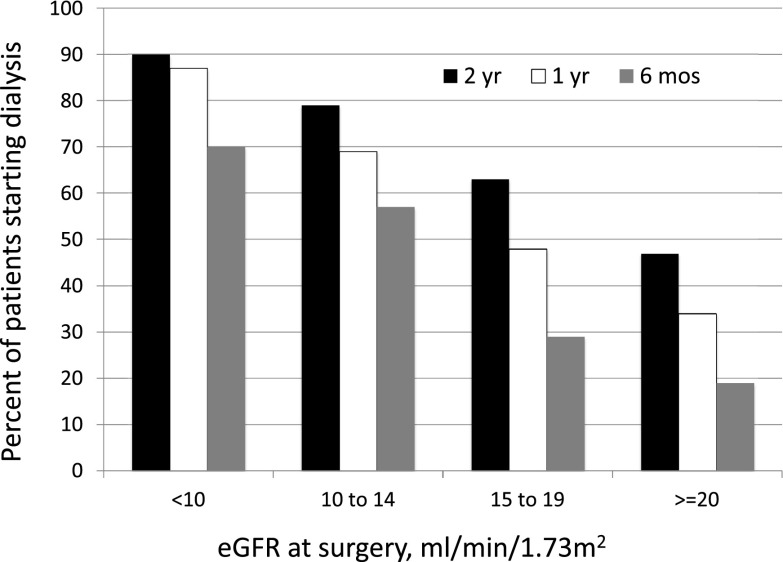
Overall, hemodialysis was initiated within 6 months, 1 year, and 2 years in 119 (39%), 175 (57%), and 211 (68%) patients, respectively. The association of predialysis eGFR at AVF surgery with initiation of dialysis is summarized in Figure 1. Among the 308 patients with CKD undergoing predialysis AVF creation, 211 (68%) initiated hemodialysis within 2 years, 47 (15%) died without starting dialysis, and 50 (16%) survived without dialysis during the 2-year follow-up. The proportion of patients who started dialysis within 2 years varied markedly by eGFR at the time of surgery (90%, 79%, 63%, and 47% of patients with eGFR<10, 10–14, 15–19, and ≥20 ml/min per 1.73 m2, respectively; P<0.001). A smaller proportion of patients initiated hemodialysis within 1 year (87%, 69%, 48%, and 34% of patients with eGFR<10, 10–14, 15–19, and ≥20 ml/min per 1.73 m2, respectively; P<0.001), and even fewer initiated dialysis within 6 months (70%, 50%, 29%, and 19% of patients with eGFR<10, 10–14, 15–19, and ≥20 ml/min per 1.73 m2, respectively; P<0.001). In summary, the likelihood of initiation of dialysis at 6 months, 1 year, or 2 years was progressively lower for patients undergoing AVF creation at an eGFR<10, 10–14, 15–19, and ≥20 ml/min per 1.73 m2, respectively (Figure 1). The mean eGFRs at dialysis initiation were 6.9±1.9, 9.2±2.2, 10.4±3.1, and 11.0±3.3 ml/min per 1.73 m2 for patients whose eGFRs at AVF surgery were <10, 10–14, 15–19, and ≥20 ml/min 1.73 m2, respectively (P<0.001).
Figure 1.
Predialysis arteriovenous fistula (AVF) placement and time to hemodialysis initiation by eGFR at AVF placement. Hemodialysis initiation was more likely if the predialysis AVF was placed at lower eGFR. Bar graphs show the likelihood of initiating hemodialysis by eGFR at AVF placement (P<0.001 between eGFR groups at 6 months, 1 year, and 2 years).
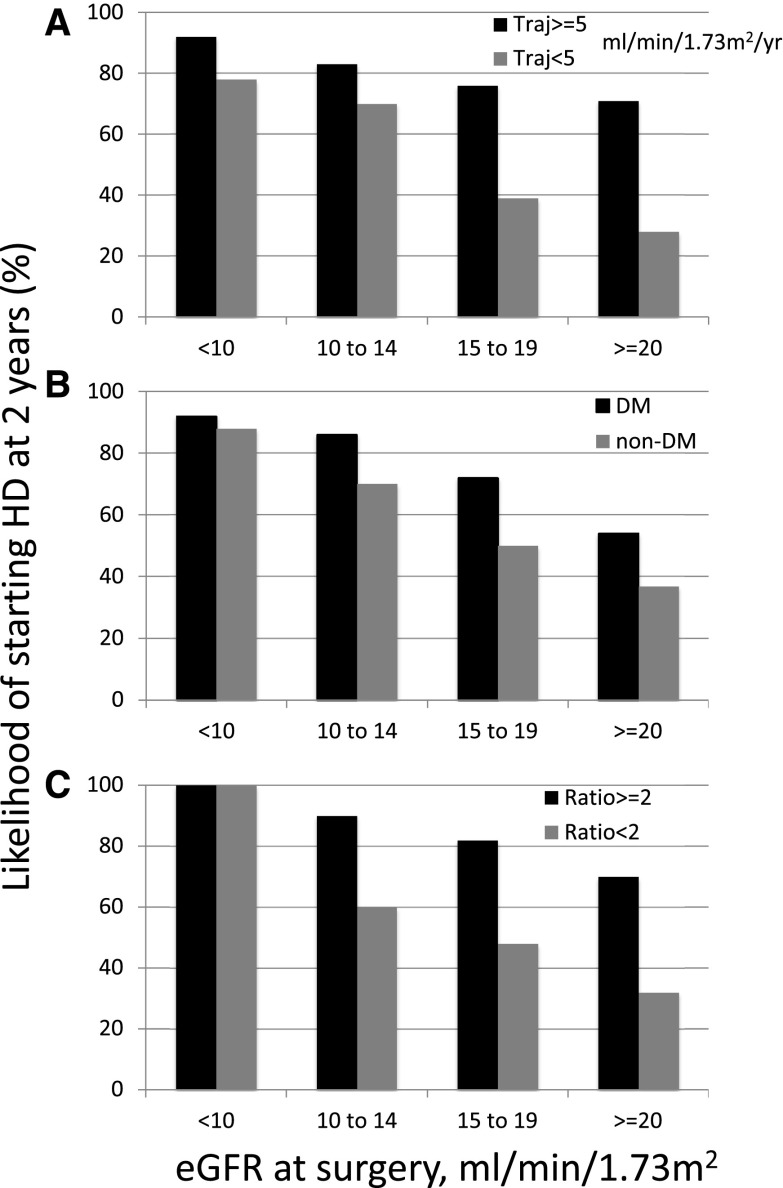
Using univariate logistic regression, a younger patient age, diabetes, black race, lower eGFR at access surgery, greater decline in eGFR over the preceding year, and greater magnitude of proteinuria were each significantly associated with a greater likelihood of starting hemodialysis within 2 years of access surgery (Table 2). Using multiple variable logistic regression analysis, four factors were associated with initiation of hemodialysis: eGFR at access surgery, diabetes, GFR trajectory in the year preceding AVF surgery, and degree of proteinuria (Table 3). For each GFR cohort, initiation of hemodialysis was more likely in patients with a faster GFR trajectory (Figure 2A). Similarly, for each GFR cohort, diabetes was associated with a higher likelihood of initiating hemodialysis (Figure 2B). Finally, for each GFR cohort, a greater magnitude of proteinuria was associated with a greater likelihood of starting dialysis within 2 years (Figure 2C). A GFR trajectory ≥5 ml/min per 1.73 m2 per year was significantly less likely in older patients (age ≥65 years old) than in younger ones (odds ratio, 0.51; 95% confidence interval [95% CI], 0.31 to 0.82; P<0.01). Strikingly, among the 145 patients with an eGFR<15 ml/min per 1.73 m2 at the time of AVF creation, 27 (19%) did not start dialysis during the ensuing 2 years. The most prominent difference between those who did not start dialysis and those who did was the greater proportion of patients with a spot urine protein-to-creatinine ratio ≤2.0 (83% versus 40%; odds ratio, 7.01; 95% CI, 2.22 to 22.14; P<0.001).
Table 2.
Univariate regression analysis of factors associated with likelihood of initiating dialysis within 2 years of predialysis arteriovenous fistula surgery
| Factor | Odds Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| Age, ≥65 versus <65 yr old | 0.54 | 0.33 to 0.89 | 0.02 |
| Sex, women versus men | 0.96 | 0.59 to 1.56 | 0.87 |
| Race, black versus white | 1.74 | 1.04 to 2.89 | 0.03 |
| Diabetes, yes versus no | 2.10 | 1.29 to 3.41 | 0.003 |
| CAD, yes versus no | 1.12 | 0.65 to 1.96 | 0.67 |
| PVD, yes versus no | 1.57 | 0.71 to 3.45 | 0.26 |
| CHF, yes versus no | 1.15 | 0.68 to 1.94 | 0.61 |
| eGFR at AVF surgery per 5 ml/min per 1.73 m2 | 0.46 | 0.34 to 0.58 | <0.001 |
| GFR trajectory per 3 ml/min per 1.73 m2 per yr | 1.15 | 1.04 to 1.26 | <0.01 |
| Urine protein-to-creatinine ratioa per 1 U | 1.49 | 1.25 to 1.77 | <0.001 |
| Systolic BP per 10 mmHg | 1.11 | 1.00 to 1.23 | 0.05 |
CAD, coronary artery disease; PVD, peripheral vascular disease; CHF, congestive heart failure; AVF, arteriovenous fistula.
Both urine protein and urine creatinine are in milligrams per deciliter.
Table 3.
Multivariable logistic regression analysis of factors associated with likelihood of initiating dialysis within 2 years of predialysis arteriovenous fistula surgery
| Factor | Odds Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| eGFR at AVF surgery per 5 ml/min per 1.73 m2 | 0.45 | 0.31 to 0.64 | <0.001 |
| Diabetes, yes versus no | 2.51 | 1.22 to 5.15 | 0.003 |
| GFR trajectory per 3 ml/min per 1.73 m2 per yr | 1.54 | 1.09 to 2.17 | 0.01 |
| Spot urine protein-to-creatinine ratioa per 1-U increase | 1.39 | 1.14 to 1.71 | <0.001 |
AVF, arteriovenous fistula.
Both urine protein and urine creatinine are in milligrams per deciliter.
Figure 2.
A faster eGFR trajectory, diabetes, and proteinuria are each associated with a higher likelihood of hemodialysis initiation within 2 years of arteriovenous fistulas (AVF) creation. Likelihood of initiation of dialysis at 2 years in patients with different eGFR values at arteriovenous fistula surgery stratified by (A) eGFR trajectory (Traj; ≥5 versus <5 ml/min per 1.73 m2 in the preceding year), (B) diabetes, and (C) magnitude of proteinuria (urine protein-to-creatinine ratio ≥2 versus <2; both urine protein and urine creatinine were expressed as milligrams per deciliter). DM, diabetes mellitus; HD, hemodialysis.
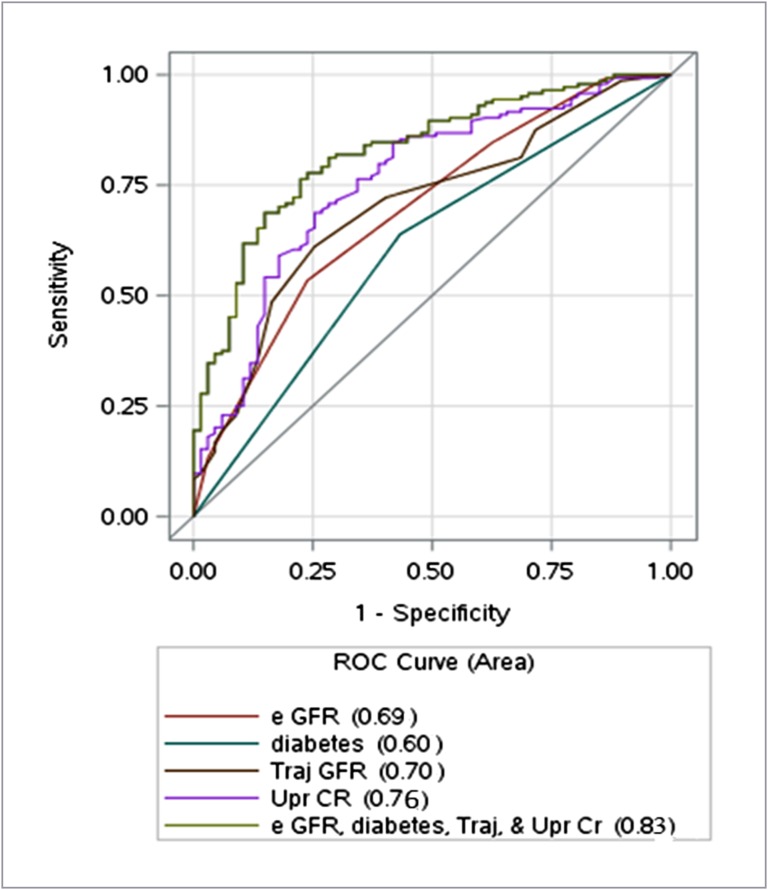
We generated ROC curves to quantify the predictive value of preoperative clinical factors at AVF surgery for the likelihood of initiating hemodialysis within 2 years. eGFR alone had a moderate predictive value for dialysis initiation (area under the curve =0.69; 95% CI, 0.63 to 0.76; P<0.001) (Figure 3). However, inclusion in the ROC curve of all four factors associated with initiation of hemodialysis in the multiple variable logistic regression model increased the area under the curve substantially to 0.83 (95% CI, 0.77 to 0.88; P<0.001).
Figure 3.
Receiver operating characteristic (ROC) curves to evaluate the predictive value of baseline clinical factors at arteriovenous fistula surgery on the likelihood of initiating hemodialysis within 2 years. For eGFR alone, area under the curve (AUC) was 0.69 (95% confidence interval [95% CI], 0.63 to 0.76; P<0.001). For the multivariable model (including eGFR at surgery, diabetes, GFR trajectory [Traj; change in eGFR during the preceding year], and proteinuria [ratio of urine protein [Upr] to urine creatinine [CR]; expressed as milligrams per deciliter]), AUC was 0.83 (95% CI, 0.77 to 0.88; P<0.001).
Likelihood of Permanent Access Use at Initiation of Hemodialysis in Patients with Predialysis AVF Placement
Among the subset of 211 patients who initiated hemodialysis within 2 years of AVF creation, only 47% used a permanent access on their first hemodialysis session. The likelihood rates of using the permanent access on starting dialysis were 30%, 57%, 44%, and 63% in patients initiating hemodialysis ≤90, 91–180, 181–360, and ≥360 days after AVF surgery, respectively (P=0.002 for ≤90 days versus >90 days; P=0.15 for patients initiating hemodialysis 91–180, 181–360, and ≥360 days after AVF surgery). The likelihood of starting hemodialysis with a permanent access was not significantly associated with patient age, sex, race, comorbidities, access location, preoperative vascular diameters, eGFR at access surgery, GFR trajectory in the year preceding access surgery, degree of proteinuria, or the immediate preoperative systolic BP (Table 4). Using multiple variable logistic regression, none of these variables predicted starting hemodialysis with a permanent access. Among patients initiating dialysis with an access, 92% were still using the access after 1 month of dialysis. Of note, even among the subset of patients with an eGFR>20 ml/min per 1.73 m2 at the time of AVF surgery, only 45% used a permanent access at initiation of dialysis.
Table 4.
Univariate regression analysis of factors associated with likelihood of using a permanent access at initiation dialysis in patients with predialysis arteriovenous fistula surgery
| Factor | Odds Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| Age, ≥65 versus <65 yr old | 1.38 | 0.75 to 2.52 | 0.30 |
| Sex, women versus men | 0.79 | 0.46 to 1.36 | 0.40 |
| Race, black versus white | 0.84 | 0.46 to 1.55 | 0.58 |
| Diabetes, yes versus no | 0.95 | 0.54 to 1.67 | 0.86 |
| CAD, yes versus no | 1.44 | 0.78 to 2.65 | 0.24 |
| PVD, yes versus no | 1.55 | 0.71 to 3.42 | 0.27 |
| CHF, yes versus no | 1.02 | 0.57 to 1.82 | 0.95 |
| Access location, forearm versus upper arm | 1.02 | 0.59 to 1.76 | 0.94 |
| Arterial diameter per mm | 0.96 | 0.72 to 1.29 | 0.80 |
| Vein diameter per mm | 1.02 | 0.83 to 1.25 | 0.86 |
| eGFR at AVF surgery per 5 ml/min per 1.73 m2 | 1.26 | 0.92 to 1.72 | 0.14 |
| GFR trajectory per 3 ml/min per 1.73 m2 per yr | 0.93 | 0.85 to 1.02 | 0.14 |
| Urine protein-to-creatinine ratioa per 1 U | 0.95 | 0.86 to 1.04 | 0.25 |
| Systolic BP per 10 mmHg | 0.96 | 0.86 to 1.08 | 0.53 |
CAD, coronary artery disease; PVD, peripheral vascular disease; CHF, congestive heart failure; AVF, arteriovenous fistula.
Both urine protein and urine creatinine are in milligrams per deciliter.
Discussion
This study evaluated the influence of kidney function and clinical characteristics at the time of pre–ESRD AVF surgery on the likelihood of initiating dialysis during follow-up. We found that initiation of dialysis was inversely related to the eGFR at AVF surgery. However, the ROC curve indicates that, used alone, eGFR at access surgery has only a moderate predictive value for initiation of dialysis. In other words, this study highlights the uncertainty in predicting time to initiation of dialysis in patients with advanced CKD when eGFR is used as the sole criterion. Thus, 21% of patients with an eGFR of 10–14 ml/min per 1.73 m2 did not start dialysis within 2 years. Conversely, 19% of patients with an eGFR≥20 ml/min per 1.73 m2 initiated dialysis within 6 months. Given the unpredictable rate of progression of kidney failure in individual patients, it is inevitable that some patients will initiate dialysis before they have undergone access surgery, whereas others will fail to progress even 2 years after their access surgery.
Selection of the optimal eGFR for access surgery requires finding the optimal balance between placing the access too early and placing the access too late. A recent computer simulation evaluated the likelihood of starting hemodialysis when AVFs were created in patients at different eGFR values (18). According to that model, the likelihood rates of starting hemodialysis after AVF creation were 80% and 90% when eGFRs at the time of surgery were <20 and 15 ml/min per 1.73 m2, respectively. This predictive model seems to be reasonably accurate, because the corresponding likelihoods of starting hemodialysis after AVF creation in this study were 74% (182 of 246) and 81% (118 of 145) in patients with eGFRs<20 and <15 ml/min per 1.73 m2, respectively.
Vascular access guidelines promulgated by different national societies have proposed various eGFR thresholds for referral of a patient with CKD to the surgeon for access creation (10–12). Unfortunately, these guidelines are opinion based, and their validity has not been subjected to empirical evaluation. This study suggests that eGFR by itself is a relatively poor predictor of the future need for dialysis. As a consequence, if we applied the Canadian guidelines (eGFR=15–20 ml/min per 1.73 m2) to our study cohort, 37% of the patients receiving an AVF would not have started dialysis in the ensuing 2 years. Even worse, applying the Fistula First or European guidelines (eGFR<30 ml/min per 1.73 m2), 85% of our patient cohort with an eGFR≥25 ml/min per 1.73 m2 receiving an AVF would not have started dialysis within 2 years. Our results suggest that optimal timing of AVF surgery in patients who are predialysis may need to take into account not just kidney function but also, other clinical variables, including diabetic status, GFR trajectory before AVF creation, and degree of proteinuria. Although age was associated with initiation of dialysis in the univariate analysis, it was no longer a significant factor in the multiple variable analysis. This discrepancy was because of the slower GFR trajectory (eGFR decrease in the year preceding AVF surgery) in older patients with CKD, and it is consistent with observations in two previous studies (7,19). The predictive value of diabetes and magnitude of proteinuria in the model is in agreement with the observation that CKD progresses faster in patients with diabetes and those with more proteinuria (20). Finally, the contribution of GFR trajectory to the predictive model is consistent with a recent investigation, which reported that both current eGFR level and the eGFR trajectory were predictors of progression to ESRD in patients with CKD (21). If our observations are confirmed in a multicenter study, they may provide a basis for a more customized approach to planning AVF placement in patients with CKD.
It was disappointing that only about one half of patients undergoing predialysis AVF creation actually initiated dialysis without a catheter, despite a reasonable time available to ensure AVF maturation. However, these findings are consistent with a recent analysis of a nationally representative United States cohort of patients with CKD undergoing predialysis AVF surgery that reported initiation of dialysis with a catheter in 46% of the cohort (22). Of interest, none of the baseline clinical factors in this study were significantly associated with the likelihood of starting hemodialysis with a mature access. In addition, whereas use of a permanent access at dialysis initiation was lower in patients with ≤3 months between AVF creation and ESRD, it was no better among patients with >6 months between AVF creation and ESRD than in those with a 3- to 6-month interval. These observations suggest that processes of care are more important than patient characteristics in determining whether the AVF is ready to use at dialysis initiation. In this regard, a previous publication from our center highlighted the complexity of and long delays in achieving this goal (23).
The strengths of this study include use of dedicated vascular access coordinators and a prospective vascular access database to ensure comprehensive patient follow-up, reliance on a small number of highly experienced access surgeons, and use of eGFR obtained on the surgery date (information that is missing from large administrative databases). In addition, this study evaluated the potential contribution of multiple clinical factors to the prediction of dialysis initiation. The study also has some limitations. First, it represents the experience of a single large dialysis center, and the observations may not generalize to some dialysis centers. It is reassuring, however, that the proportion of patients with pre–ESRD AVF surgery initiating hemodialysis within 2 years (68%) was remarkably similar to that (67%) reported recently for a nationally representative cohort of patients with CKD (22). Of course, it will be important to validate our observations at other dialysis centers. Second, an eGFR on the surgery date was missing for 17% of the patients undergoing pre–ESRD access surgery. If this subset differed substantially from those with available eGFR values, this might skew the results somewhat. However, the similarities in age, sex, diabetes, and comorbidities between the excluded cohort and the study cohort suggest that exclusion of these patients did not substantially affect the findings.
In summary, the results of this study suggest that optimizing the timing of AVF creation in patients predialysis requires consideration of kidney function, diabetic status, GFR trajectory (eGFR decline in the preceding year), and degree of proteinuria. If our observations can be validated in a multicenter study, they may form the basis of an enhanced tool to optimize the timing of AVF creation in patients predialysis.
Disclosures
T.L. is a consultant for Proteon Therapeutics (Waltham, MA). M.A. is a consultant for CorMedix (Bedminster, NJ) and Gore (Flagstaff, AZ).
Acknowledgments
T.L. is supported by an American Society of Nephrology Carl W. Gottschalk Research Scholar grant, the University of Alabama Nephrology Research Center Anderson Innovation Award, and University of Alabama at Birmingham Center for Clinical and Translational Science Multidisciplinary Pilot Award grant 1UL1TR001417-01. M.A. is supported by grant R01DK085027 from the National Institute of Diabetes, Digestive and Kidney Diseases. A.A.B. received partial funding for this manuscript from National Institute of Health grant T32 DK007545.
Portions of this manuscript were presented at the American Society of Nephrology Kidney Week Meeting, November 4–8, 2015 in San Diego, California.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Can We Predict the Unpredictable after Vascular Access Creation?,” on pages 1729–1731.
References
- 1.Collins AJ, Foley RN, Gilbertson DT, Chen SC: The state of chronic kidney disease, ESRD, and morbidity and mortality in the first year of dialysis. Clin J Am Soc Nephrol 4[Suppl 1]: S5–S11, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Xue JL, Dahl D, Ebben JP, Collins AJ: The association of initial hemodialysis access type with mortality outcomes in elderly Medicare ESRD patients. Am J Kidney Dis 42: 1013–1019, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Lacson E Jr., Wang W, DeVries C, Leste K, Hakim RM, Lazarus M, Pulliam J: Effects of a nationwide predialysis educational program on modality choice, vascular access, and patient outcomes. Am J Kidney Dis 58: 235–242, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Lacson E Jr., Wang W, Lazarus JM, Hakim RM: Change in vascular access and mortality in maintenance hemodialysis patients. Am J Kidney Dis 54: 912–921, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Xue H, Lacson E Jr., Wang W, Curhan GC, Brunelli SM: Choice of vascular access among incident hemodialysis patients: A decision and cost-utility analysis. Clin J Am Soc Nephrol 5: 2289–2296, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradbury BD, Chen F, Furniss A, Pisoni RL, Keen M, Mapes D, Krishnan M: Conversion of vascular access type among incident hemodialysis patients: Description and association with mortality. Am J Kidney Dis 53: 804–814, 2009 [DOI] [PubMed] [Google Scholar]
- 7.O’Hare AM, Bertenthal D, Walter LC, Garg AX, Covinsky K, Kaufman JS, Rodriguez RA, Allon M: When to refer patients with chronic kidney disease for vascular access surgery: Should age be a consideration? Kidney Int 71: 555–561, 2007 [DOI] [PubMed] [Google Scholar]
- 8.ESRD NCC National Coordinating Center: Fistula First Catheter Last Initiative. Available at: http://esrdncc.org/ffcl/. Accessed June 19, 2015
- 9.KDOQI: KDOQI clinical practice guidelines and clinical practice recommendations for vascular access. Am J Kidney Dis 48[Suppl 1]: S176–S322, 2006 [DOI] [PubMed]
- 10.Canadian Society of Nephrology (CSN) : Report of the Canadian Society of Nephrology Vascular Access Working Group. Semin Dial 25: 22–25, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Ohira S, Naito H, Amano I, Azuma N, Ikeda K, Kukita K, Goto Y, Sakai S, Shinzato T, Sugimoto T, Takemoto Y, Haruguchi H, Hino I, Hiranaka T, Mizuguchi J, Miyata A, Murotani N; Japanese Society for Dialysis Therapy : 2005 Japanese Society for Dialysis Therapy guidelines for vascular access construction and repair for chronic hemodialysis. Ther Apher Dial 10: 449–462, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Tordoir J, Canaud B, Haage P, Konner K, Basci A, Fouque D, Kooman J, Martin-Malo A, Pedrini L, Pizzarelli F, Tattersall J, Vennegoor M, Wanner C, ter Wee P, Vanholder R: EBPG on Vascular Access. Nephrol Dial Transplant 22[Suppl 2]: ii88–ii117, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Allon M, Bailey R, Ballard R, Deierhoi MH, Hamrick K, Oser R, Rhynes VK, Robbin ML, Saddekni S, Zeigler ST: A multidisciplinary approach to hemodialysis access: Prospective evaluation. Kidney Int 53: 473–479, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Allon M, Lockhart ME, Lilly RZ, Gallichio MH, Young CJ, Barker J, Deierhoi MH, Robbin ML: Effect of preoperative sonographic mapping on vascular access outcomes in hemodialysis patients. Kidney Int 60: 2013–2020, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Robbin ML, Gallichio MH, Deierhoi MH, Young CJ, Weber TM, Allon M: US vascular mapping before hemodialysis access placement. Radiology 217: 83–88, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Robbin ML, Chamberlain NE, Lockhart ME, Gallichio MH, Young CJ, Deierhoi MH, Allon M: Hemodialysis arteriovenous fistula maturity: US evaluation. Radiology 225: 59–64, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Singh P, Robbin ML, Lockhart ME, Allon M: Clinically immature arteriovenous hemodialysis fistulas: Effect of US on salvage. Radiology 246: 299–305, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Shechter SM, Skandari MR, Zalunardo N: Timing of arteriovenous fistula creation in patients with CKD: A decision analysis. Am J Kidney Dis 63: 95–103, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Oliver MJ, Quinn RR, Garg AX, Kim SJ, Wald R, Paterson JM: Likelihood of starting dialysis after incident fistula creation. Clin J Am Soc Nephrol 7: 466–471, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruggenenti P, Perna A, Gherardi G, Benini R, Remuzzi G: Chronic proteinuric nephropathies: Outcomes and response to treatment in a prospective cohort of 352 patients with different patterns of renal injury. Am J Kidney Dis 35: 1155–1165, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Kovesdy CP, Coresh J, Ballew SH, Woodward M, Levin A, Naimark DM, Nally J, Rothenbacher D, Stengel B, Iseki K, Matsushita K, Levey AS; CKD Prognosis Consortium : Past decline versus current eGFR and subsequent ESRD risk [published online ahead of print December 11, 2015]. J Am Soc Nephrol doi:ASN.2015060687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee T, Thamer M, Zhang Y, Zhang Q, Allon M: Outcomes of elderly patients after predialysis vascular access creation. J Am Soc Nephrol 26: 3133–3140, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee T, Barker J, Allon M: Tunneled catheters in hemodialysis patients: Reasons and subsequent outcomes. Am J Kidney Dis 46: 501–508, 2005 [DOI] [PubMed] [Google Scholar]