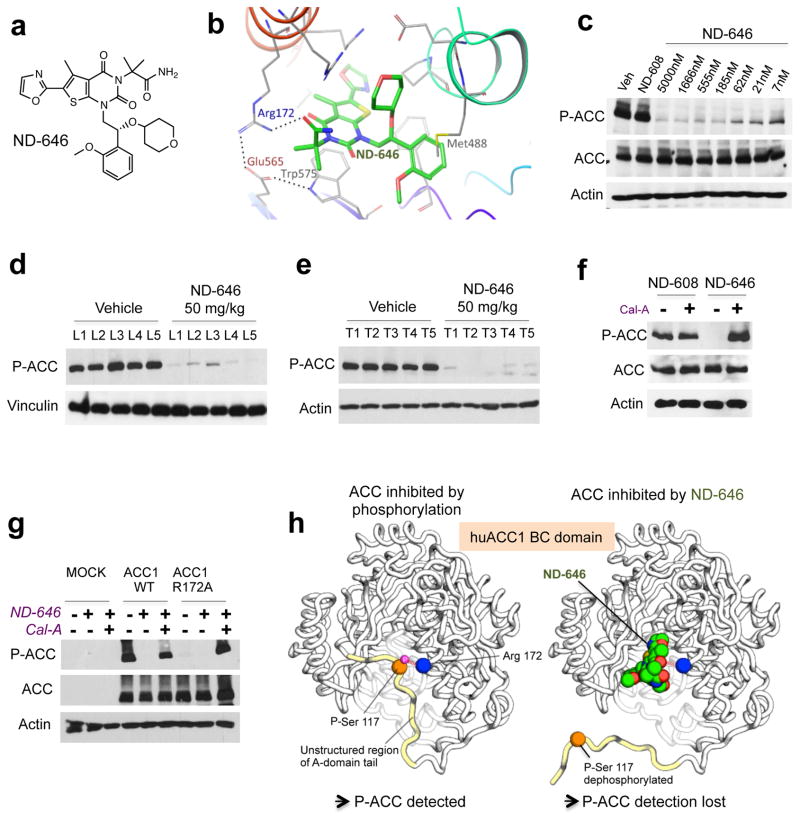
Figure 2. Properties of ND-646, a small molecule allosteric inhibitor of ACC. AMPK phosphorylation sites can be used as a biomarker to monitor ACC engagement by ND-646.
(a) Chemical structure of ND-646.
(b) Model of ND-646 bound to the BC domain of human ACC1. Image depicts a docked pose of ND-646 and ACC1 derived from co-crystal structures of ND-646 complexed with huACC2.
(c) P-ACC detection in A549 cells treated for 24 hrs with 5000 nM ND-608 or a dose response of ND-646.
(d) P-ACC detection in livers of FVB/n mice and (f) KrasG12D/+; p53−/ lung tumors in mice treated orally with a single dose of vehicle or 50 mg/kg ND-646 for 3 hrs. Numbers represent individual samples from separate mice (n=5 per treatment).
(f) P-ACC detection in A549 cells co-treated with either 500 nM ND-608 or ND-646 ± Calyculin A (Cal-A) for 1 hr.
(g) P-ACC detection in ACC1-KO HEK293 cells transiently expressing Mock, ACC1 wild-type or ACC1 phosphopeptide binding mutant (ACC1R172A). Cells were treated with 500 nM ND-646 ± Cal-A.
(h) Model describing the mechanism of ACC inhibition by ND-646 and the P-ACC biomarker. Identical effects occur in ACC2-BC at the conserved residues.
For (c–g) data are representative from one of at least two separate experiments.