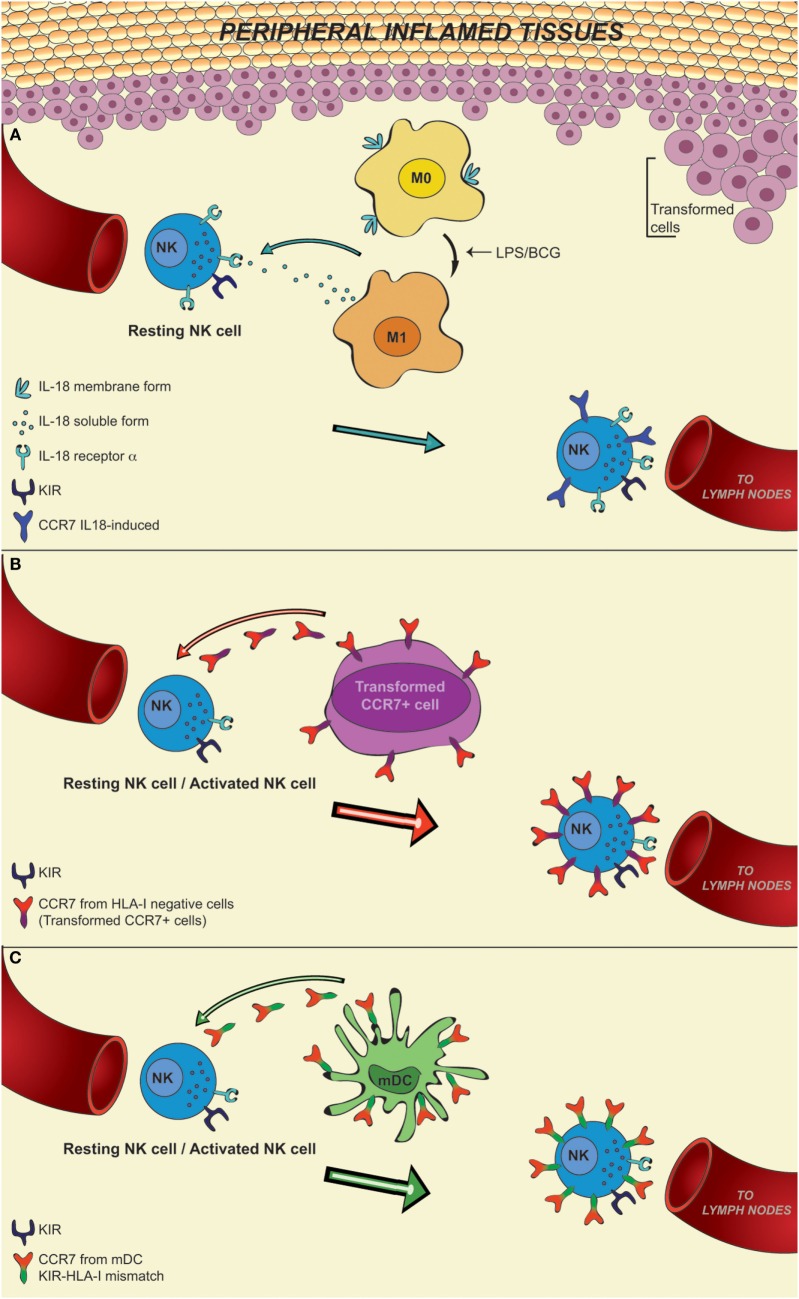
Figure 1.
In this figure, it is shown that CD56dim KIR+ NK cells, after recruitment from blood into inflamed peripheral tissues in response to chemokine gradients, may de novo express CCR7, a chemokine receptor able to confer immune cells with the ability to migrate to lymph nodes. This event may occur in a microenvironment rich in IL-18, a pro-inflammatory cytokine released by M0 macrophages during differentiation toward M1 following activation. This de novo expression can be induced only on a fraction of NK cells characterized by the expression of the IL-18Rα (resting NK cells), whereas it cannot be induced on activated NK cells that express little IL-18Rα (A). Otherwise, the expression of CCR7 on CD56dim NK cells can be induced by a mechanism of trogocytosis on either resting or activated NK cells, regardless of their level of IL-18Rα expression. This mechanism is finely controlled by the specific interaction between KIRs on NK cells and HLA class I molecules on CCR7+ cells. In particular, inhibitory KIRs block this transfer, whereas some activating KIRs are able to strongly promote the CCR7 acquisition by NK cells. This means that in an autologous setting, NK cells may acquire CCR7 only by interacting with HLA-I negative CCR7+ cells, such as transformed cells (B). In contrast, in an allogeneic setting, as in haplo-HSCT, characterized by KIR/HLA class I mismatch, KIR+ alloreactive NK cells can express CCR7 when they interact with allogeneic CCR7+ mDCs (C).