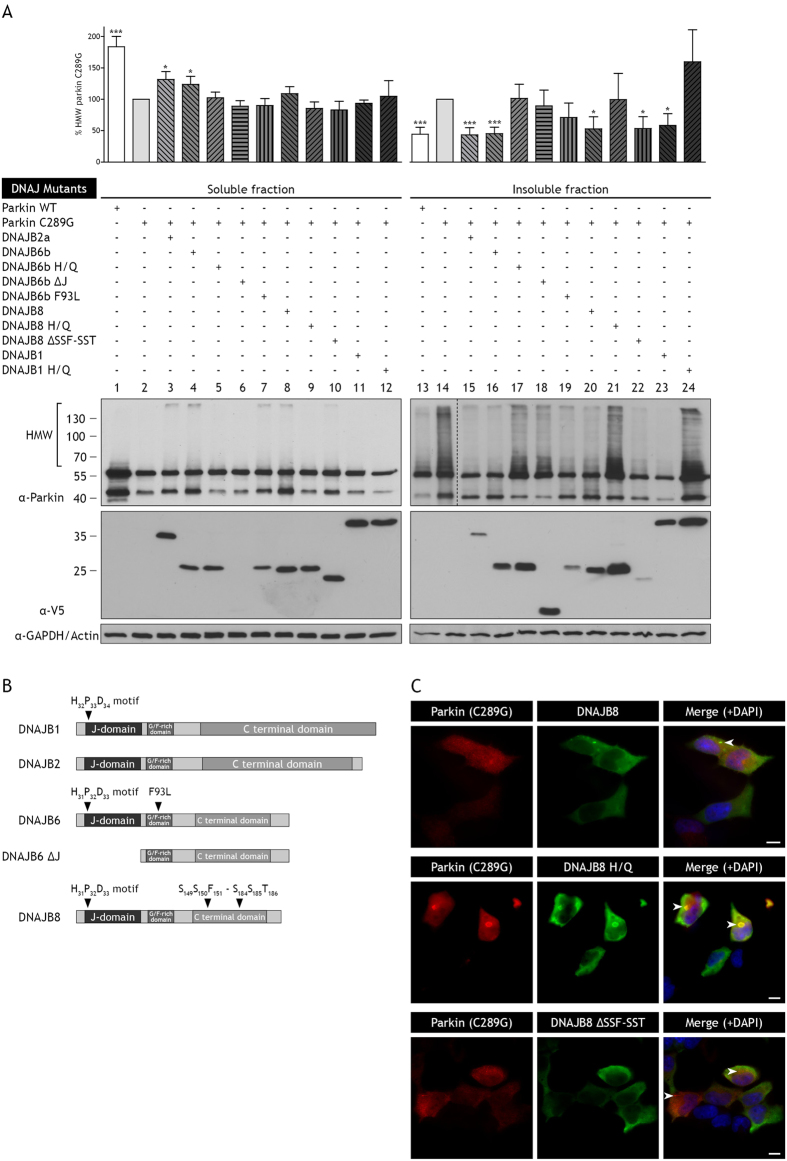
Figure 2. The anti-aggregation activity of DNAJ chaperones is dependent on the J-domain, not on the SSF-SST domain.
(A) Aggregation of parkin C289G is prevented when co-transfected with DNAJB2a, DNAJB6b, DNAJB8, or DNAJB1. Mutations in the J-domain of the chaperones (H/Q) and deletion of the J-domain as a whole (ΔJ), results in the loss of prevention of aggregation of parkin C289G. DNAJB6b with a mutation in the G/F-rich region (F93L) retains its anti-aggregation activity. Deletion of the SSF-SST domain (ΔSSF-SST) of DNAJB8 does not affect its ability to prevent aggregation. Blots are analysed for parkin C289G high molecular weight species and normalised to parkin C289G (*p < 0.05; ***p < 0.001; n > 4 independent samples, mean ± SEM). (B) Schematic overview of DNAJB1, DNAJ2a, DNAJB6, and DNAJB8 and the mutants. (C) Representative immunofluorescence pictures of cells co-transfected with flag-tagged parkin C289G (red) and V5-tagged chaperones (green). DAPI staining is shown in blue. Bar represents 10 μm. Co-transfection with DNAJB8 shows a reduction in parkin C289G aggregates and co-localization of DNAJB8 with parkin C289G, indicated by arrowheads. Co-transfection with DNAJB8 H/Q still shows formation of parkin C289G aggregates mainly concentrated into large perinuclear inclusions, indicated by arrowheads. Parkin C289G and DNAJB8 H/Q co-localize but aggregate formation is not prevented. Co-transfection with DNAJB8 ΔSSF-SST reveals clearance of parkin C289G aggregates and in occasional small aggregations co-localization of DNAJB8 ΔSSF-SST with parkin, indicated by arrowheads.