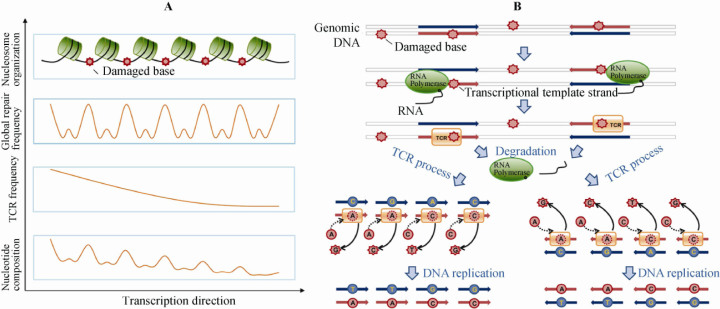
Figure 7.
Schematic illustration for the proposed mechanism of TCR-associated mutations and sequence variation-defined periodicity. A. Mutation-derived periodicity is a result of nucleosome positioning. Nucleosomes are regularly positioned near the transcription starts along genes. Since the linker DNA is exposed and more vulnerable to mutagenesis, it is damaged more frequently, repaired more often, and therefore leaves more mutations resulting from TCR. In addition, the mutation frequencies are higher at the 5′ end of highly expressed EIGs, leading to a descending mutation gradient toward the 3′ end. B. After transcription initiation, DNA damage occurring on the template (transcribed) strand results in stall and degradation of RNA polymerase II (RNAPII) when TCR displaces RNAPII and initiates the DNA repair process. In the process of repair, due to the low-fidelity of DNA polymerase, A and C are often added regardless of the damaged bases, leading to A or C mismatches at the damaged loci. After one round of DNA replication, mutations are fixed in the daughter cells. Since TCR is asymmetric, only the template strand is repaired, and the resulting distribution of mutations would also be asymmetric. Therefore, the strong asymmetry of the four major mutation types (C→T, A→G, C→G and G→T) is easily explained in such a way; C→T is most prevalent as it represents a transition rather than a transversion like C→G and G→T, while the size difference between purine and pyrimidine may explain why the frequency of A→G is much lower than C→T.