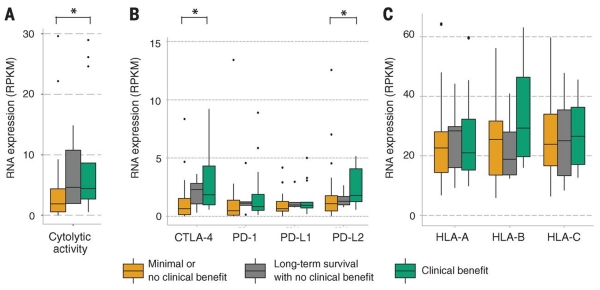
Fig. 3. Immune microenvironment cytolytic and immune activity correlates with response to ipilimumab.
(A) Patients who achieved clinical benefit from immune checkpoint blockade therapy had significantly higher levels of tumor cytolytic activity than those who had minimal or no benefit from ipilimumab (P = 0.039). (B) Patients who achieved clinical benefit from ipilimumab therapy had significantly higher levels of expression immune checkpoint receptors than those who did not (CTLA-4: P = 0.033, PD-L2: P = 0.041). One point is not shown because of an outlying high CTLA-4 expression value in a nonresponder patient (>50 reads per kilobase per million mapped reads). (C) Response to ipilimumab did not correlate with expression of or mutations in HLA alleles (P > 0.05 for all). Asterisks (*) indicate P < 0.05.