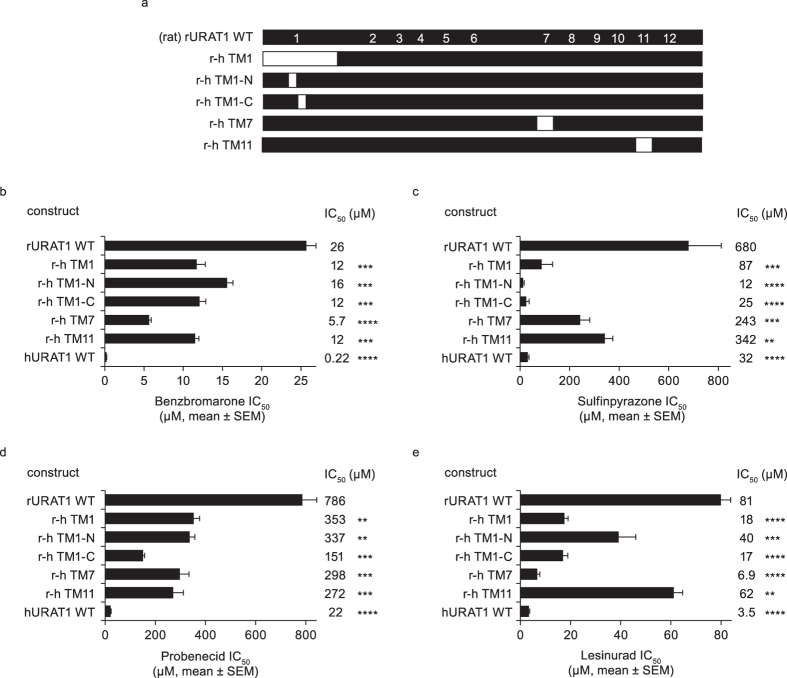
Figure 3. Chimeras of human (h) URAT1 and rat (r) URAT1 reveal that TMS 1, 7 and 11 of hURAT1 confer high affinity responses to inhibitors.
(a) Diagram of rURAT1 wild type (rURAT1 WT, black box) with the predicted secondary structures showing the approximate location of the twelve transmembrane (TM) segments (numbered), the large first extracellular loop (EC1) and the large third intracellular loop (IC3), and diagrams of individual rat-to-human (r–h) chimeras where small regions of rURAT1 were replaced with human URAT1 (hURAT1, white boxes). (b–e) Potencies against rURAT1 and the rat-to human (r-h) chimeras shown in (a) for benzbromarone (b), sulfinpyrazone (c), probenecid (d), and lesinurad (e). Dose-response curves for each construct were performed as in Fig. 1, and results are the mean ± SEM from at least three experiments. Asterisks indicate a significant difference in the mean value from rURAT1 (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001).