Figure 6.
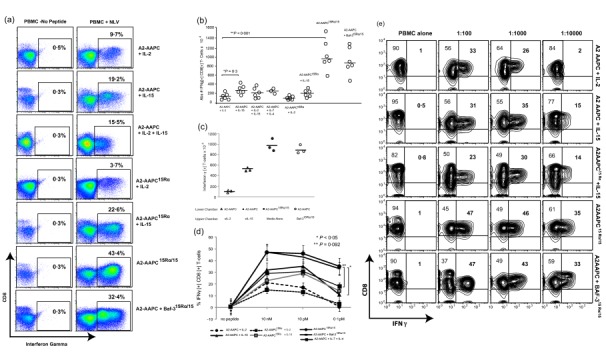
15Rα/15 complexes support the generation of high‐avidity antigen‐specific T cells. The proportion of CD8+ interferon (IFN)‐γ+ T cells responding to the cytomegalovirus (CMV)pp65 epitope NLVPMVATV (NLV) presented by human leucocyte antigen (HLA) A 02:01 were quantitated on day 21 for each parallel culture condition. (i) A2‐artificial antigen‐presenting cells (AAPCs)+ soluble interleukin (sIL)−2 (20U/ml) or sIL‐15 (10 ng/ml), sIL‐2 + sIL‐15 or sIL‐7 (10 ng/ml) + sIL‐4 (1666 U/ml); (ii) A2‐AAPCIL‐15Rα + sIL‐2 or sIL‐15; and (iii) A2‐AAPC15Rα/15 or A2‐AAPC + Baf‐3IL‐15Rα/IL‐15, with no exogenous cytokines. Aliquots of autologous peripheral blood mononuclear cells (PBMC) were loaded (37ºC × 3 h) with serial dilutions of NLV peptide (10 nM, 10 pM, 0·1pM), and co‐incubated with T cells at a responder : target ratio of 5 : 1 × 12 h in the presence of brefeldin A (BFA). T cells were labelled with immunofluorescent antibodies against CD3, CD4, CD8, fixed and then permeabilized (fix and perm kit; Invitrogen) and then incubated with anti‐human IFN‐γ fluorescein isothiocyanate (FITC). Data were acquired on a BD LSRII flow cytometer and analysed using FlowJo software. (a) One representative example demonstrating the proportion of IFN‐γ+ CD8+ T cells in response to 10 nM peptide‐loaded targets within CD3+ T cells is shown. (b) The total yield of IFN‐γ+ CD8+ T cells generated in response to 10 nM peptide was calculated from the percentage of IFN‐γ+ CD8+ T cells and plotted for each donor in each culture condition. (c) T cells from three separate HLA A2 + donors that were sensitized in six‐well Transwell plates according to cytokine conditions providing sIL‐2, sIL‐15 or 15Rα/15 complexes via the permeable transmembrane. Antigen‐specific T cells generating functional cytokines in response to 10 nM NLV peptide were evaluated on day 21 to quantitate the proportion of NLV‐specific CD8+ IFN‐γ+ T cells. (d) After 21 days of stimulation, the proportion of IFN‐γ+ CD8+ T cells elicited upon secondary stimulation with autologous targets loaded with serial dilutions of NLV peptide is shown for each donor in each culture condition (error bars = standard error of the mean), and (e) in one representative donor, IFN‐γ+ CD8+ T cells elicited in response targets loaded with serial peptide dilutions is shown. The proportion IFN‐γ+ CD8+ T cells in 15Rα/15‐stimulated T cells was significantly greater than sIL‐2 or sIL‐15 cultures at all peptide dilutions (P = 0·001). There was a significant reduction in the proportion of IFN‐γ+ CD8+ T cells at 10 pM versus 0·1 pM peptide concentrations for sIL‐15 cultures (P < 0·05).
