Figure 6.
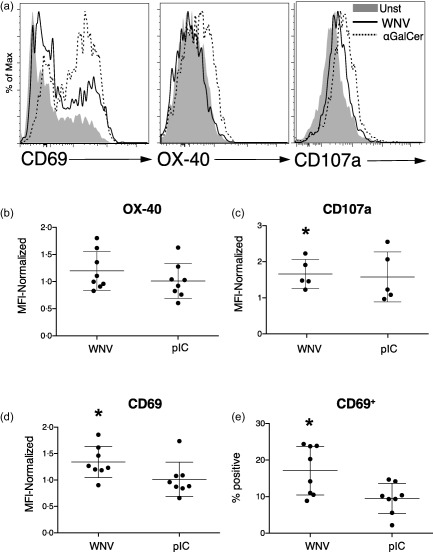
West Nile virus (WNV)‐infected dendritic cells (DCs) activate invariant natural killer T cells (iNKTs). DCs were left unstimulated, infected with WNVKUN at multiplicity of infection (MOI) = 5, incubated with the CD1d ligand α‐galactosylceramide (αGalCer) (100 ng/ml) or stimulated with poly‐inosine‐cytosine (pIC) (50 μg/ml) for 24 h. DCs (10 000/well) were then co‐cultured with iNKTs (50 000/well) that had been isolated from blood, expanded in vitro and rested. Purified iNKTs were CD3+ and bound the CD1d‐PBS−57 tetramer. (a) After 48 h, iNKT expression of CD69, OX‐40 and CD107a [lysosomal‐associated membrane protein 1 (LAMP1)] after incubation with unstimulated (Unst), WNV‐infected or αGalCer‐exposed DCs was determined by flow cytometry. (b,d) The surface expression of OX‐40, CD107a and CD69 on iNKTs in multiple experiments is compiled. In each experiment, mean fluorescence intensities (MFIs) on iNKTs incubated with stimulated (WNV or pIC) DCs were normalized to expression on iNKTs incubated with unstimulated DCs. (e) The percentage of CD69+ iNKTs is shown. The mean percentage of CD69+ iNKTs after exposure to unstimulated DCs was 10·1%. Symbols represent individual experiments with unique donor DC and iNKT combinations. Significance was evaluated using a repeated‐measure (RM) one‐way analysis of variance (anova) followed by a Dunnett's multiple comparison test (comparing each stimulated value to the corresponding unstimulated value). *P < 0·05.
