Fig. 7.
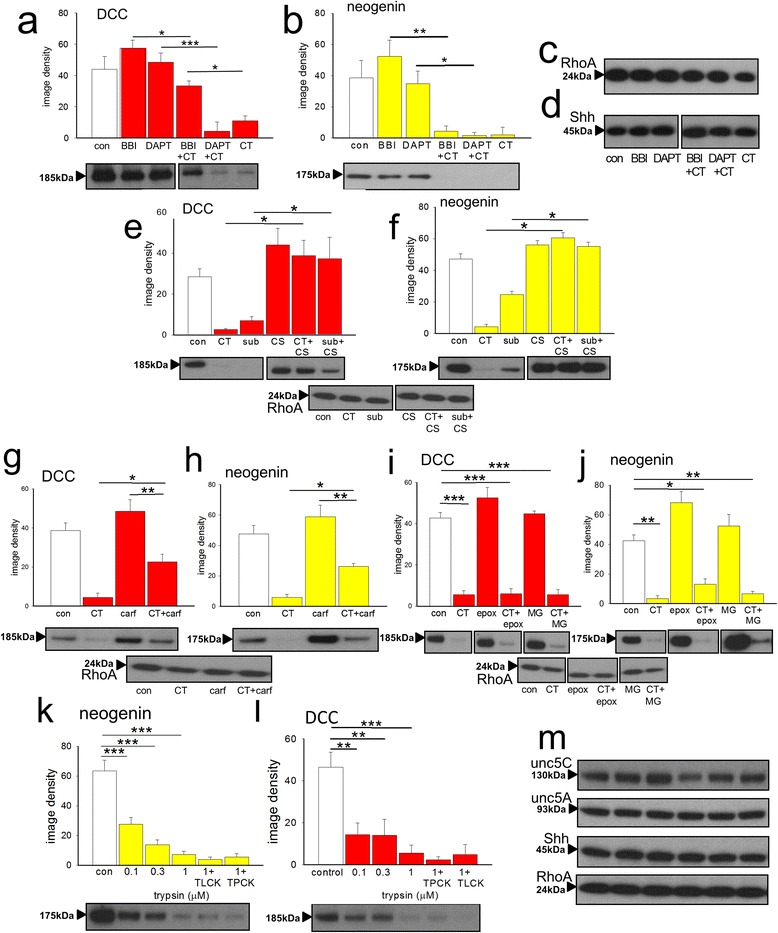
Potential inhibitors of serine proteases. Protein expression in extracts of brain slices is summarised as image densities (arbitrary units) of Western blots quantified using Image J for the effects of chymotrypsin (CT, 1 μM) on DCC (a), neogenin (b), RhoA (c) and Shh (d) expression. Sample blots are shown below each chart, which illustrate the concentration-dependent effects of the proteases. The Bowman-Birk soybean inhibitor (BBI, 100 μM) blocked the effect of chymotrypsin on DCC expression (a) although the γ-secretase inhibitor DAPT (5 μM) did not prevent the loss of DCC (c) or neogenin (d). Neither BBI nor DAPT had any effect themselves on protein expression. Chymostatin (CS, 30 μM) had no significant effect alone but blocked the inhibitory effects of both chymotrypsin and subtilisin on DCC (e) and neogenin (f) expression. The chymotryptic proteasome inhibitor carfilzomib (carf, 50nM) significantly reduced the effect of chymotrypsin (CT, 1 μM) on DCC (g) and neogenin (h) expression. However, two other inhibitors of the 20S proteasome, epoxomicin (epox, 1 μM) and MG132 (MG, 10 μM) had no significant effect themselves and did not block the effect of chymotrypsin on DCC (i) or neogenin (j). Trypsin reduced the expression of neogenin (k) and DCC (l) with no significant effects on unc5H3, unc5H1, Shh and RhoA expression (m) but these effects were not prevented by TPCK or TLCK. Sample blots are shown below each chart. Bars represent mean ± s.e.mean (n = 4). *P < 0.05; **P < 0.01; ***P < 0.001 relative to the control bar
