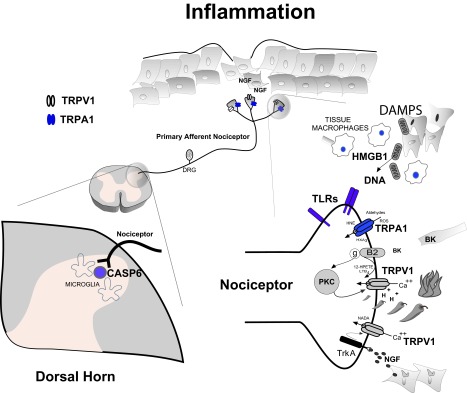
Figure 1. Inflammatory Pain.
Tissue injury evokes a complex series of cellular responses that together is proposed to drive painful hyperalgesic states. Specialized primary afferent nociceptors (top center) innervate tissues and signal potential or actual cellular injury through detection of noxious chemical, thermal and mechanical stimuli. Electrochemical transduction of noxious stimuli at nociceptor terminals include activation of transient receptor potential (TRP) ion channel family members. As a result of the synthesis and/or release of injury – induced inflammatory products, nociceptor transducing elements may be positively modulated or directly activated driving painful and hyperalgesic states. A number of these products (eg: peptides [BK], activation of PKC, TrkA activation by NGF, acid [H +], lipoxygenase products - 12-HPETE, LTB 4, NADA, as well as reactive oxygen species [ROS], aldehydes, HNE and HXA 3) have been shown to either modulate or activate TRPV1 and TRPA1 respectively (bottom right). Certain products of inflammation (eg: nerve growth factor [NGF], ROS, aldehydes) modulate multiple pain transducing receptors/elements. Depending on the mechanism and severity of tissue injury, innate immune cell responses will be recruited. Damage-associated molecular patterns (DAMPs) such as HMGB1 and mitochondrial derived DNA bind and activate toll-like receptors (TLRs) expressed on nociceptor terminals further driving hyperalgesia. Monocyte derived macrophages invade injured tissue and release a complex array of cytokines, chemokines and growth factors such as NGF. Together, they conspire to transform nociceptor phenotype to pathophysiologic states of persistent nociceptor activation, lowered firing thresholds and/or exaggerated response properties. Tissue inflammation also influences the central processing of nociceptive input in the dorsal horn of the spinal cord (bottom left). As a result, central nociceptor terminals upregulate and release signaling molecules such as CASP6 that activates microglia – dependent inflammatory hyperalgesia.