Figure 2.
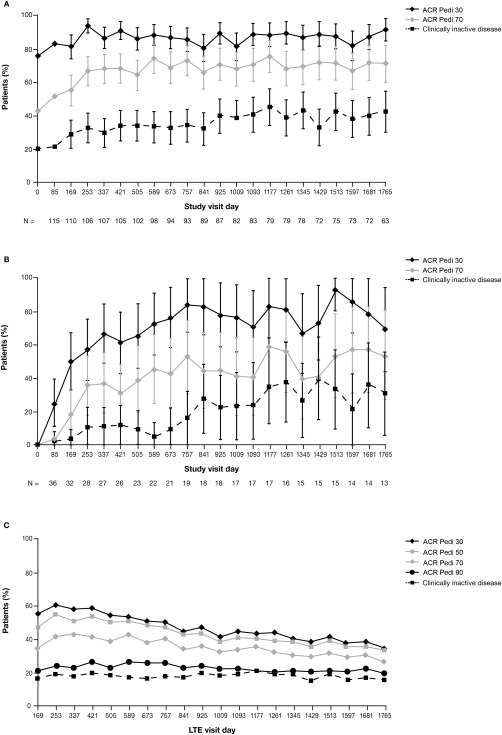
Proportions of juvenile idiopathic arthritis patients meeting the American College of Rheumatology (ACR) Pediatric 30 (Pedi 30), Pedi 50, Pedi 70, and Pedi 90 criteria for improvement and the proportions achieving an inactive disease status over time. A, Combined group of patients who received abatacept during the 6‐month double‐blind withdrawal period and those who received placebo during the 6‐month double‐blind withdrawal period. B, Patients who did not achieve an ACR Pedi 30 response during the initial 4‐month open‐label lead‐in period and who entered the long‐term extension (LTE) phase directly. In A and B, data were derived from as‐observed analyses based on all patients who entered the open‐label LTE. Bars show the 95% confidence intervals. n = number of patients with available data on each visit day. C, Intent‐to‐treat population. ACR Pedi responses throughout the LTE phase were also evaluated in a post hoc intent‐to‐treat analysis based on all 190 patients who had entered the lead‐in phase of the trial, with any patient who discontinued at any point considered a nonresponder. A total of 76 patients remained in the study and had efficacy data available on day 1,765.
