Abstract
AIMS: To study in vivo human lens epithelial cell proliferation on the anterior surface of PMMA implants and its interaction with postoperative blood-aqueous barrier breakdown in eyes undergoing cataract surgery. METHODS: A prospective study was carried out on three consecutive patient cohorts undergoing cataract surgery with intraocular lens implantation using three different surgical techniques which produce different anatomical relations between the implant and lens capsule. Specular microscopy of the anterior implant surface was used to document the natural history, topography, and density of lens epithelial cells and the laser flare and cell meter were used to measure postoperative blood-aqueous barrier breakdown. RESULTS: All groups showed lens epithelial cell proliferation onto the anterior surface of PMMA implants. This was initiated by and restricted to the region of anterior capsule-implant contact and decreased with the onset of anterior capsular opacification. Significant correlation was found in all groups between lens epithelial cell proliferation and postoperative blood-aqueous barrier breakdown. CONCLUSIONS: Human lens epithelial cell behaviour on PMMA surfaces in vivo differs from that seen in culture studies. Humoral factors in the aqueous, biomaterial properties of the implant, and its anatomical relations with the anterior and posterior lens capsule influence lens epithelial cell behaviour in vivo.
Full text
PDF

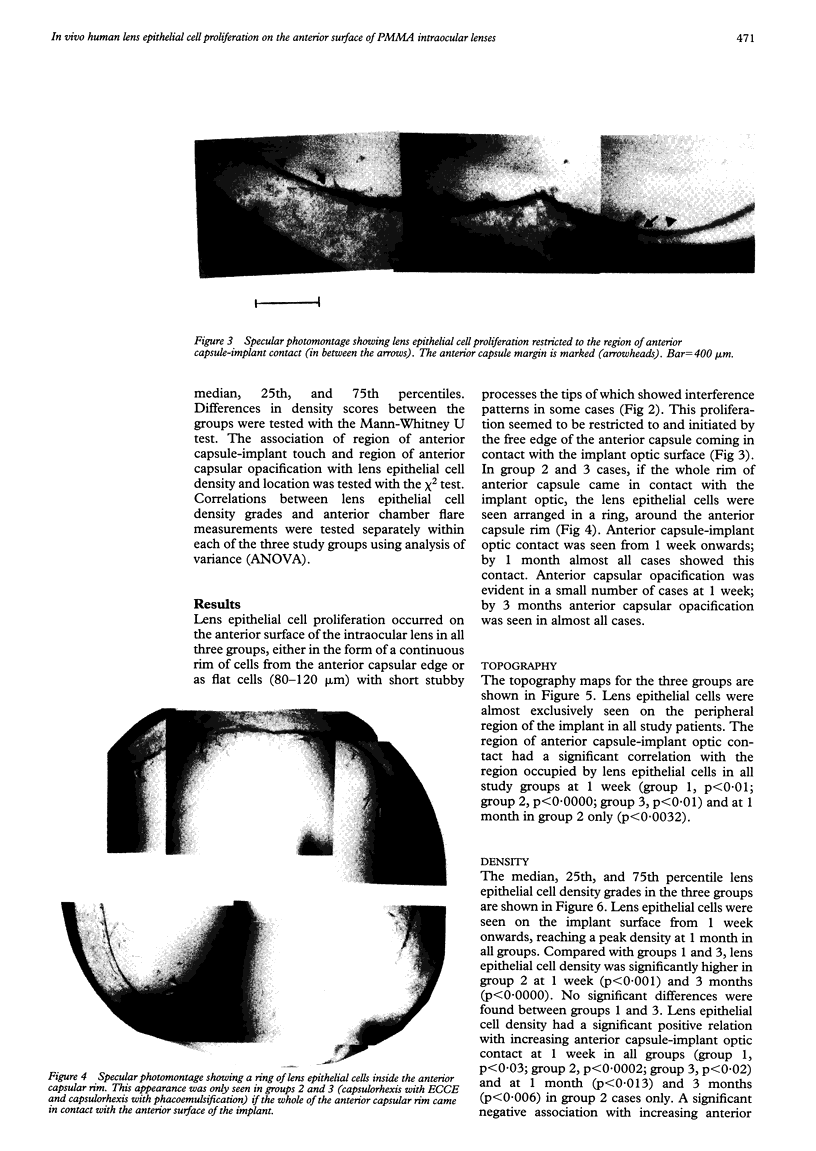
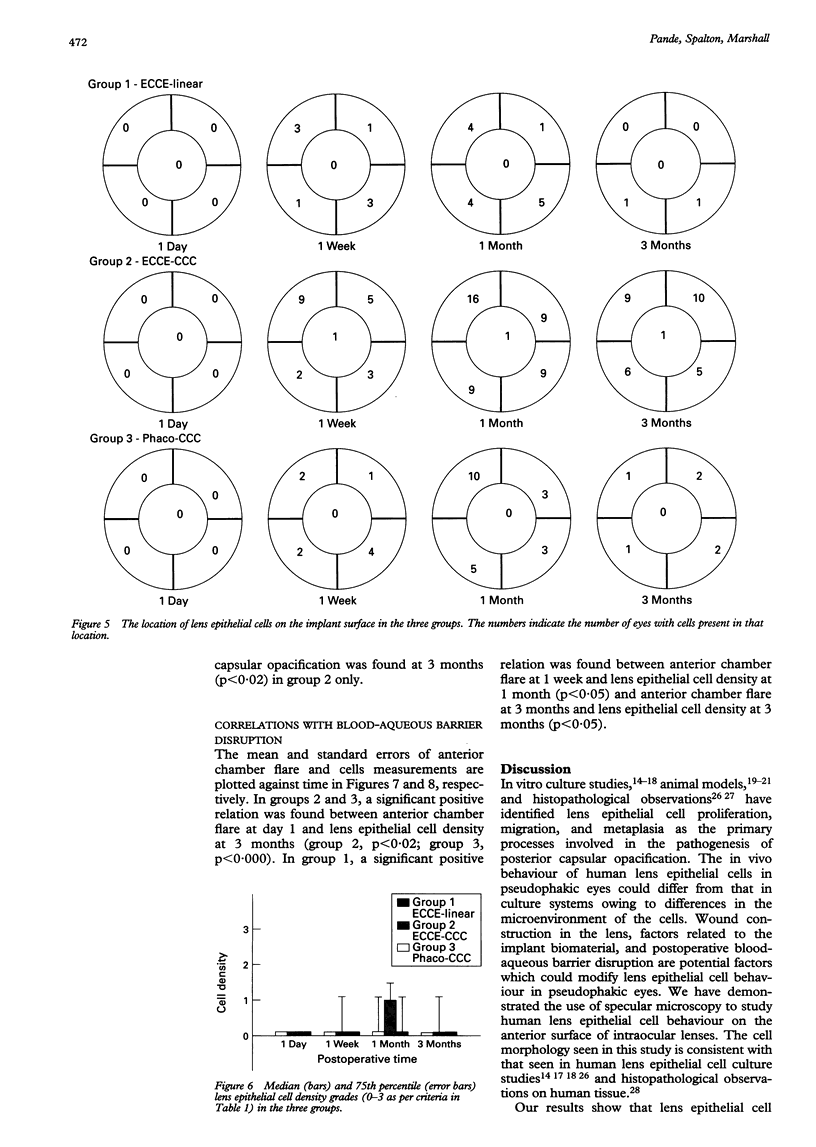
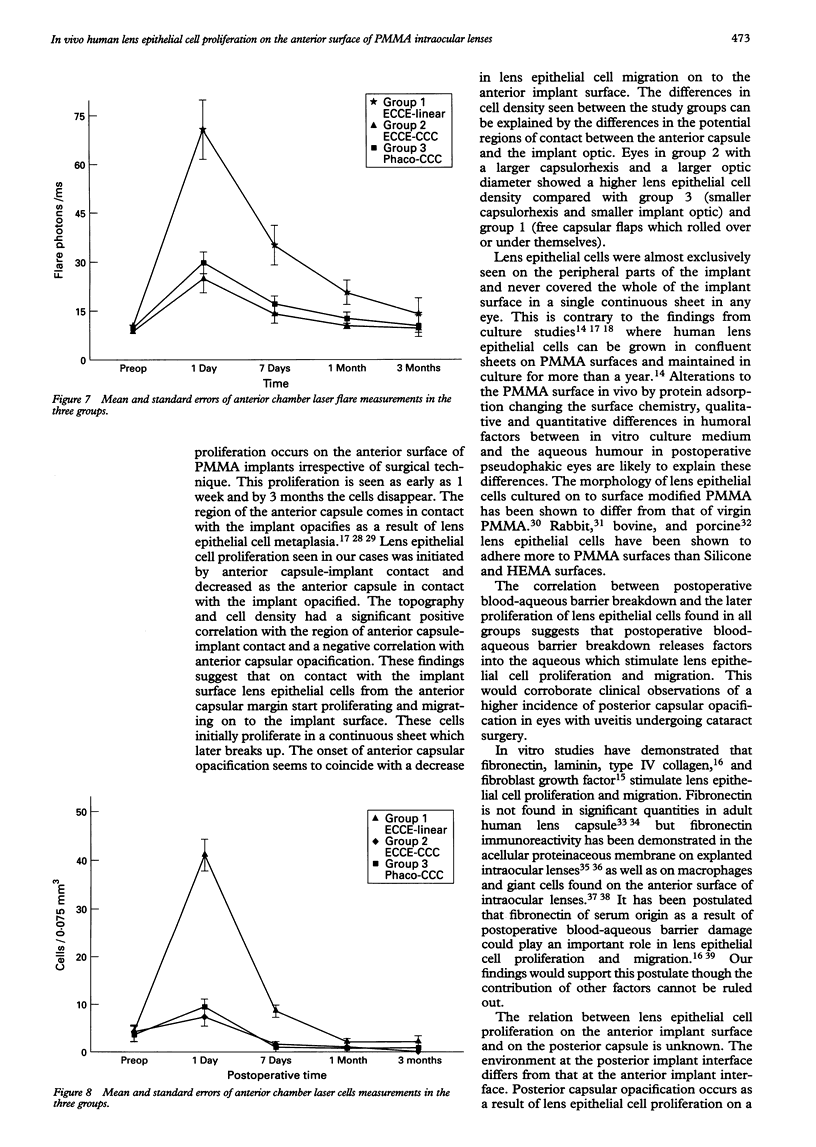

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apple D. J., Mamalis N., Loftfield K., Googe J. M., Novak L. C., Kavka-Van Norman D., Brady S. E., Olson R. J. Complications of intraocular lenses. A historical and histopathological review. Surv Ophthalmol. 1984 Jul-Aug;29(1):1–54. doi: 10.1016/0039-6257(84)90113-9. [DOI] [PubMed] [Google Scholar]
- Arita T., Murata Y., Lin L. R., Tsuji T., Reddy V. N. Synthesis of lens capsule in long-term culture of human lens epithelial cells. Invest Ophthalmol Vis Sci. 1993 Feb;34(2):355–362. [PubMed] [Google Scholar]
- Ayaki M., Kyu N. [Histopathologic study of after-cataract in the pseudophakic rabbit eye using in-the-bag fixation. (II)]. Nippon Ganka Gakkai Zasshi. 1990 Jun;94(6):559–565. [PubMed] [Google Scholar]
- Born C. P., Ryan D. K. Effect of intraocular lens optic design on posterior capsular opacification. J Cataract Refract Surg. 1990 Mar;16(2):188–192. doi: 10.1016/s0886-3350(13)80728-6. [DOI] [PubMed] [Google Scholar]
- Boyd W., Peiffer R. L., Siegal G., Eifrig D. E. Fibronectin as a component of pseudophakic acellular membranes. J Cataract Refract Surg. 1992 Mar;18(2):180–183. doi: 10.1016/s0886-3350(13)80928-5. [DOI] [PubMed] [Google Scholar]
- Cobo L. M., Ohsawa E., Chandler D., Arguello R., George G. Pathogenesis of capsular opacification after extracapsular cataract extraction. An animal model. Ophthalmology. 1984 Jul;91(7):857–863. doi: 10.1016/s0161-6420(84)34225-7. [DOI] [PubMed] [Google Scholar]
- Cunanan C. M., Tarbaux N. M., Knight P. M. Surface properties of intraocular lens materials and their influence on in vitro cell adhesion. J Cataract Refract Surg. 1991 Nov;17(6):767–773. doi: 10.1016/s0886-3350(13)80409-9. [DOI] [PubMed] [Google Scholar]
- Fagerholm P., Fitzsimmons T., Härfstrand A., Schenholm M. Reactive formation of hyaluronic acid after small and large lens injury. Acta Ophthalmol Suppl. 1992;(205):58–64. doi: 10.1111/j.1755-3768.1992.tb02182.x. [DOI] [PubMed] [Google Scholar]
- Frezzotti R., Caporossi A., Mastrangelo D., Hadjistilianou T., Tosi P., Cintorino M., Minacci C. Pathogenesis of posterior capsular opacification. Part II: Histopathological and in vitro culture findings. J Cataract Refract Surg. 1990 May;16(3):353–360. doi: 10.1016/s0886-3350(13)80708-0. [DOI] [PubMed] [Google Scholar]
- Hansen T. E., Otland N., Corydon L. Posterior capsule fibrosis and intraocular lens design. J Cataract Refract Surg. 1988 Jul;14(4):383–386. doi: 10.1016/s0886-3350(88)80143-3. [DOI] [PubMed] [Google Scholar]
- Hara T., Azuma N., Chiba K., Ueda Y., Hara T. Anterior capsular opacification after endocapsular cataract surgery. Ophthalmic Surg. 1992 Feb;23(2):94–98. [PubMed] [Google Scholar]
- Hara T., Hara T., Kojima M., Nakaizumi H., Yamamura T., Sasaki K. Specular microscopy of the anterior lens capsule after endocapsular lens implantation. J Cataract Refract Surg. 1988 Sep;14(5):533–540. doi: 10.1016/s0886-3350(88)80012-9. [DOI] [PubMed] [Google Scholar]
- Hettlich H. J., Wenzel M., Janssen M., Mittermayer C. Immunohistochemische Untersuchungen der menschlichen Linsenkapsel. Fortschr Ophthalmol. 1990;87(2):147–149. [PubMed] [Google Scholar]
- Humphry R. C., Ball S. P., Brammall J. E., Conn S. J., Rich W. J. Lens epithelial cells adhere less to HEMA than to PMMA intraocular lenses. Eye (Lond) 1991;5(Pt 1):66–69. doi: 10.1038/eye.1991.13. [DOI] [PubMed] [Google Scholar]
- Ichijima H., Kobayashi H., Ikada Y. In vitro evaluation of biocompatibility of surface-modified poly(methyl methacrylate) plate with rabbit lens epithelial cells. J Cataract Refract Surg. 1992 Jul;18(4):395–401. doi: 10.1016/s0886-3350(13)80079-x. [DOI] [PubMed] [Google Scholar]
- Kanagawa R., Saika S., Ohmi S., Tamura M., Nakao T. Presence and distribution of fibronectin on the surface of implanted intraocular lenses in rabbits. Graefes Arch Clin Exp Ophthalmol. 1990;228(5):398–400. doi: 10.1007/BF00927249. [DOI] [PubMed] [Google Scholar]
- Kappelhof J. P., Pameyer J. H., De Jong P. T., Jongkind J. F., Vrensen G. F. The proteinaceous coating and cytology of implant lenses in rabbits. Am J Ophthalmol. 1986 Dec 15;102(6):750–758. doi: 10.1016/0002-9394(86)90403-4. [DOI] [PubMed] [Google Scholar]
- Lovicu F. J., McAvoy J. W. Localization of acidic fibroblast growth factor, basic fibroblast growth factor, and heparan sulphate proteoglycan in rat lens: implications for lens polarity and growth patterns. Invest Ophthalmol Vis Sci. 1993 Nov;34(12):3355–3365. [PubMed] [Google Scholar]
- Lundgren B., Jonsson E., Rolfsen W. Secondary cataract. An in vivo model for studies on secondary cataract in rabbits. Acta Ophthalmol Suppl. 1992;(205):25–28. [PubMed] [Google Scholar]
- Martin R. G., Sanders D. R., Souchek J., Raanan M. G., DeLuca M. Effect of posterior chamber intraocular lens design and surgical placement on postoperative outcome. J Cataract Refract Surg. 1992 Jul;18(4):333–341. doi: 10.1016/s0886-3350(13)80067-3. [DOI] [PubMed] [Google Scholar]
- McDonnell P. J., Zarbin M. A., Green W. R. Posterior capsule opacification in pseudophakic eyes. Ophthalmology. 1983 Dec;90(12):1548–1553. doi: 10.1016/s0161-6420(83)34350-5. [DOI] [PubMed] [Google Scholar]
- Nishi K., Nishi O. [Tissue culture of human lens epithelial cells. Part II: Suppressive effect of diclofenac sodium on their proliferation and metaplasia]. Nippon Ganka Gakkai Zasshi. 1991 Jun;95(6):581–590. [PubMed] [Google Scholar]
- Nishi O., Nishi K., Hikida M. Removal of lens epithelial cells following loosening of the junctional complex. J Cataract Refract Surg. 1993 Jan;19(1):56–61. doi: 10.1016/s0886-3350(13)80282-9. [DOI] [PubMed] [Google Scholar]
- Nishi O., Nishi K. Intercapsular cataract surgery with lens epithelial cell removal. Part III: Long-term follow-up of posterior capsular opacification. J Cataract Refract Surg. 1991 Mar;17(2):218–220. doi: 10.1016/s0886-3350(13)80253-2. [DOI] [PubMed] [Google Scholar]
- Nishi O. Removal of lens epithelial cells by ultrasound in endocapsular cataract surgery. Ophthalmic Surg. 1987 Aug;18(8):577–580. [PubMed] [Google Scholar]
- Ohara K. Biomicroscopy of surface deposits resembling foreign-body giant cells on implanted intraocular lenses. Am J Ophthalmol. 1985 Mar 15;99(3):304–311. doi: 10.1016/0002-9394(85)90360-5. [DOI] [PubMed] [Google Scholar]
- Ohara K., Itakura K., Ibaraki N. Anterior capsule opacification: a cell culture model. Acta Ophthalmol Suppl. 1992;(205):29–33. doi: 10.1111/j.1755-3768.1992.tb02178.x. [DOI] [PubMed] [Google Scholar]
- Olivero D. K., Furcht L. T. Type IV collagen, laminin, and fibronectin promote the adhesion and migration of rabbit lens epithelial cells in vitro. Invest Ophthalmol Vis Sci. 1993 Sep;34(10):2825–2834. [PubMed] [Google Scholar]
- Saika S., Uenoyama S., Kanagawa R., Tamura M., Uenoyama K. Phagocytosis and fibronectin of cells observed on intraocular lenses. Jpn J Ophthalmol. 1992;36(2):184–191. [PubMed] [Google Scholar]
- Sakamoto T., Ishibashi T., Sugai S., Ohnishi Y., Fukushima M., Sueishi K. [Immunohistochemical study of the surface membrane on a intra-ocular lens]. Nippon Ganka Gakkai Zasshi. 1990 Nov;94(11):1074–1078. [PubMed] [Google Scholar]
- Sievers H., von Domarus D. Foreign-body reaction against intraocular lenses. Am J Ophthalmol. 1984 Jun;97(6):743–751. doi: 10.1016/0002-9394(84)90507-5. [DOI] [PubMed] [Google Scholar]
- Sterling S., Wood T. O. Effect of intraocular lens convexity on posterior capsule opacification. J Cataract Refract Surg. 1986 Nov;12(6):655–657. doi: 10.1016/s0886-3350(86)80080-3. [DOI] [PubMed] [Google Scholar]
- Tetz M. R., O'Morchoe D. J., Gwin T. D., Wilbrandt T. H., Solomon K. D., Hansen S. O., Apple D. J. Posterior capsular opacification and intraocular lens decentration. Part II: Experimental findings on a prototype circular intraocular lens design. J Cataract Refract Surg. 1988 Nov;14(6):614–623. doi: 10.1016/s0886-3350(88)80028-2. [DOI] [PubMed] [Google Scholar]
- Wenzel M., Reim M., Heinze M., Böcking A. Cellular invasion on the surface of intraocular lenses. In vivo cytological observations following lens implantation. Graefes Arch Clin Exp Ophthalmol. 1988;226(5):449–454. doi: 10.1007/BF02170007. [DOI] [PubMed] [Google Scholar]
- Wilhelmus K. R., Emery J. M. Posterior capsule opacification following phacoemulsification. Ophthalmic Surg. 1980 Apr;11(4):264–267. [PubMed] [Google Scholar]





