Background:
It is difficult to completely resect huge anterior chest wall keloids and then close the wound directly. We report here our retrospective analysis of our case series of patients with such keloids who underwent reconstruction with internal mammary artery perforator (IMAP) pedicled propeller flaps and then received postoperative high-dose-rate superficial brachytherapy.
Methods:
All consecutive patients with large/severe keloids on the anterior chest wall who underwent keloid resection followed by reconstruction with IMAP-pedicled propeller flaps and then high-dose-rate superficial brachytherapy in our academic hospital were identified. All cases were followed for >18 months. Donor site position, perforator pedicle, flap size, angle of flap rotation, complications, and recurrence were documented.
Results:
There were nine men and one woman. The average age was 37.9 years. The average follow-up duration was 28.7 months. The largest flap was 16 × 4 cm. The dominant perforators of the internal mammary artery were located in the sixth (n = 2), seventh (n = 5), eighth (n = 1), and ninth (n = 2) intercostal spaces. Twelve months after surgery, patients reported marked relief from keloid-associated pain and itching, except in two patients who underwent partial keloid resection; their remaining keloids were still troublesome but after conservative therapies, including steroid ointments/plasters, the keloids gradually ameliorated. Eighteen months after surgery, there was no keloid recurrence or new development of keloids on the donor site.
Conclusions:
IMAP-pedicled propeller flaps transfer skin tension from the anterior chest wall to the abdomen. Our series suggests that this approach combined with radiation therapy can control keloid recurrence.
INTRODUCTION
Keloids are cutaneous pathological scars that are characterized by chronic inflammation of the reticular layer of the dermis.1 The anterior chest wall is particularly frequently subject to the development of keloids.2 Such keloids are largely initiated by folliculitis and/or acne. A possible explanation for the high frequency with which this area develops keloids is that it is subjected to cyclical stretching force due to the daily movements of the upper arms. Recent basic research has shown that such repetitive stretching of the edges of chest wounds induces continuous inflammation of the dermis and is an important cause of heavy scarring.1–4 There are also other factors apart from such local factors that promote keloid development, including genetic factors such as single nucleotide polymorphisms5,6 and systemic factors such as hypertension (high blood pressure)7 and estrogen levels.8,9 Thus, anterior chest wall keloids are the result of several complex factors and are therefore difficult to treat.
In recent times, several effective and safe surgical approaches that additionally employ postoperative radiation treatment have been described.10,11 However, international unified guidelines remain to be developed. These studies show that small anterior chest wall keloids can be treated successfully and safely by complete surgical resection and postoperative radiation therapy. However, it is difficult to completely resect huge anterior chest wall keloids and then directly close the wound. In such cases, reconstructive methods such as tissue expansion, skin grafting, or flap transfer should be considered.
However, skin grafting tends to generate secondary contractures and tissue expansion mandates a staged operation. This makes it difficult to know when to start radiation therapy. For these reasons, we reconstruct anterior chest wall keloids by using selected local flaps. Given that perforator-pedicled propeller flaps were recently identified all over the body,12–14 we have used internal mammary artery perforator (IMAP) pedicled propeller flaps to reconstruct anterior chest wall keloids. This method has the advantage that only a single operation is needed, long and reliable flaps can be harvested safely in a short period of time, and postoperative radiation therapy can be started within a week of the operation. Moreover, such flaps exhibit natural expansion after surgery and this reduces the tension on the wound edges over time. However, one disadvantage is that both the donor and recipient sites must be irradiated to prevent postoperative keloid formation.
In this article, we present our recent case series of ten consecutive patients with anterior chest wall keloids who underwent reconstruction by this method and were then followed up for at least 18 months. The results suggest that the benefits of this approach for the patients markedly outweigh the possibility that the flap donor site develops keloids.
METHODS
Patients
All patients consented in writing to undergo the surgery and postoperative radiation therapy and for their results to be reported. The study was performed according to the principles of the Declaration of Helsinki and its revisions. All consecutive patients with large/severe keloids on the anterior chest wall who underwent reconstruction with IMAP-pedicled propeller flaps followed by postoperative radiation therapy with high-dose-rate superficial brachytherapy (HDR-SB) between April 2010 and March 2012 were identified retrospectively. All cases were followed up for at least 18 months. The donor site position, the perforator pedicle, the flap size, the angle of flap rotation, the complications, and recurrences were extracted from the medical records.
Treatment Methods
Preoperative Perforator Detection and Design of the Flap
If there was enough time, the perforators were identified before surgery by using 64-row multidetector computed tomography (MDCT), followed by image reformatting using the maximum intensity projection technique and the volume-rendering technique. All perforators could be detected with this approach. Candidate perforators, namely those with diameters greater than 1.0 mm, were marked on the patient’s skin according to the coordinate data from the MDCT. In addition, both the locations where these vessels penetrate the deep fascia and the course of the suprafascial perforator branches were traced on the computer (Fig. 1). This generated elliptically shaped flaps that followed the perforator course. If there was not enough time to perform MDCT, the perforators were identified by Doppler ultrasound only. Although the position of the perforator pedicle could be detected, it was more difficult to identify the suprafacial perforator course with this method. In these cases, the flap was designed as an elliptical shape that ran in the transverse direction of the costal bone lining. As much as possible, the flap was designed on the inframammary fold because this made it easy to close the donor site. However, in cases where the keloid was located on the cranial side, the flap was designed slightly on the cranial side of the inframammary fold.
Fig. 1.
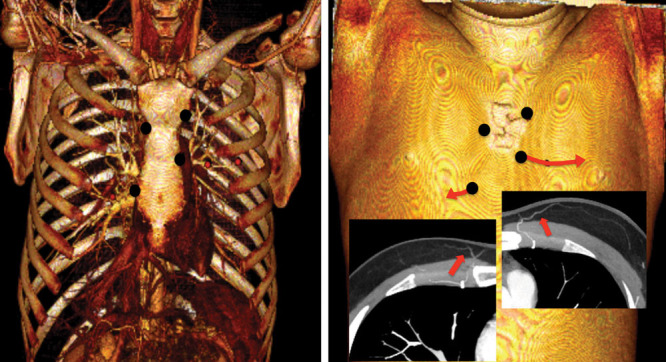
Preoperative search for perforators by 64-row multidetector computed tomography. Candidate perforators (diameter >1.0 mm) were marked. In addition, both the location where these vessels penetrated the deep fascia and the course of the suprafascial perforator branches were traced on the computer. This approach generated elliptically shaped flaps that ran along the perforator course.
The number of flaps (harvested from one side or bilaterally), the flap size, and the flap length were planned on the basis of the size of the keloid and whether inclusion cysts were present. If inclusion cysts were present and were causing repeated infections, the infected region was excised completely.
Intraoperative Methods
The keloids were first excised to the depth of the layer between the deep fascia and the superficial fascia. Thereafter, elevation of the flap from the distal portion started. The flap was harvested under the fascia because including the fascia in the flap meant that these fascia could be sutured to the surrounding deep fascia on the recipient site after transfer. The marked perforator was then found by careful dissection by curved scissors. After confirming that the flap could be rotated smoothly to fit with the recipient site, the donor site was closed. At both recipient and donor sites, the deep fascia were sutured by using 0 or 2-0 polydioxanon sutures. Thereafter, the superficial fascial layer was sutured by using 3-0 polydioxanon sutures, followed by suturing of the dermis by using 4-0 polydioxanon sutures. The superficial layer was then closed by using 6-0 polypropylene sutures. A suction drain was placed under the flap and the donor site. Nonadhesive wound dressing material such as Mepitel One (Mölnlycke Health Care US, Norcross, GA) was applied and the operation was finished.
Postoperative Care and Radiation Therapy
After confirming that the bleeding had stopped, the suction drain was removed 3–4 days after surgery. The entire region, including both the donor and recipient sites, was washed with saline and closed with polyurethane film Tegaderm (3M US, St. Paul, MN). Shortly thereafter, HDR-SB was delivered by using a remote after-loading system from an Ir-192 source. Thus, the 3-mm-thick plastic tube applicator was placed on the skin in a Q shape that included both the donor and recipient sites and matched the surgical wound shape. The applicator was fixed with adhesive tape and a 5-mm-thick spacer was placed between the skin and the applicator. A dose evaluation point was established 2 mm under the skin surface and 20 Gy was delivered in four daily fractions. About 10 days after surgery, the film dressing was removed and all of the sutures were removed. Silicone-tape fixation to stabilize the wounds at both the donor and recipient sites was then started. Such stabilization was continued for at least 6 months. All patients were followed up by scheduled visits every 3–6 months.
RESULTS
Ten patients with large anterior chest wall keloids underwent resection followed by reconstruction with IMAP-pedicled propeller flaps and then HDR-SB during the study period. There were nine men and one woman and the average age was 37.9 (range: 22–55) months. The causes of the keloids were acne and folliculitis. All keloids were extremely large (larger than 40 cm2). All cases were followed up on average for 28.7 (range: 18–42) months. In five, the perforators were identified by MDCT. In the remaining five, the perforators were found by Doppler ultrasound.
The largest flap was 16 × 4 cm (cases 5 and 7) (Table 1). Thus, the length:width ratio of the flap was 4:1. Despite this, there were no flap complications such as epithelial necrosis on the distal area. This indicates that a 4:1 length:width flap ratio is safe for this flap. One patient (case 4) received two flaps. Two patients underwent partial resection only (cases 4 and 7). The dominant perforators of the IMA were the sixth (n = 2), seventh (n = 5), eighth (n = 1), and ninth (n = 2) intercostal spaces. All perforators ran from the parasternal region in the direction of the lateral and caudal regions. The average angle of flap rotation was 108 degrees (range: 75–135 degrees). In cases of 75- or 90-degree rotation (n = 5), skeletonizing of the vascular pedicle was not necessary. However, when the angle was 120 or 135 degrees, skeletonizing was necessary for smooth rotation of the flap (n = 5). In all cases where the vascular pedicle was skeletonized, the flap did not show any ischemia or congestion after surgery. There were no surgical complications.
Table 1.
Demographic, Surgical, and Recurrence Variables of the Patients in This Study
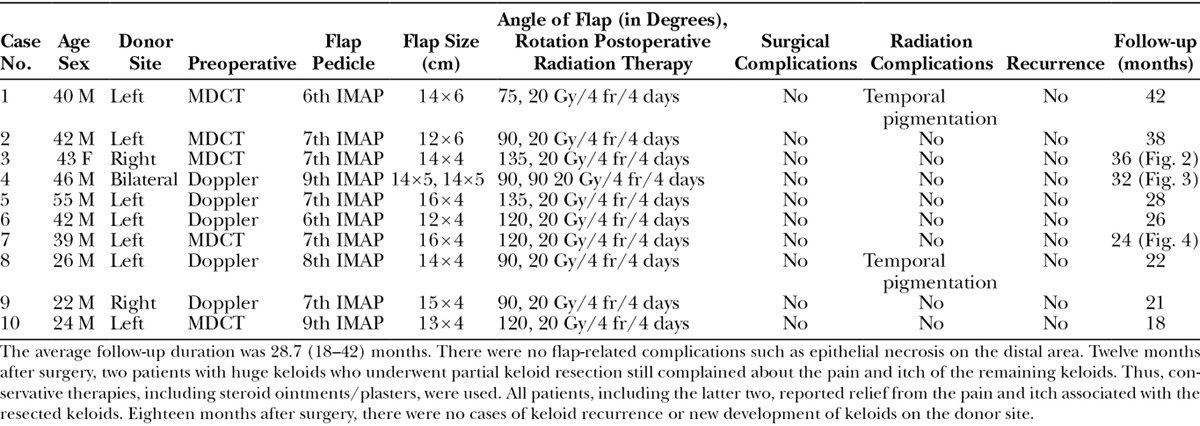
In all cases, the suction drain was successfully removed within 3–4 days of surgery and radiation could be started from 5 days of surgery without any complications. After removing the sutures, two patients exhibited pigmentation that was caused by radiation dermatitis. However, it disappeared within 3 months. There were no cases of wound dehiscence during follow-up.
Twelve months after surgery, all patients reported relief from the severe pain and itch associated with their keloids. However, the two patients who had particularly huge keloids and who underwent partial keloid resection only still complained about the pain and itch arising from the remaining keloids. Thus, conservative therapies, including steroid ointments/plasters, were used in these cases. Eighteen months after surgery, none of the resected keloids had recurred and none of the donor sites developed new keloids. The patients who were followed up for more than 18 months also did not report keloid recurrence or new development of keloids on the donor site. Three examples of the patients are shown in Figures 2–4. Cases 4 and 7 (Figs. 3, 4) are the patients who underwent partial keloid resection. At 32 and 24 months after surgery, respectively, their remaining keloids had improved significantly.
Fig. 2.
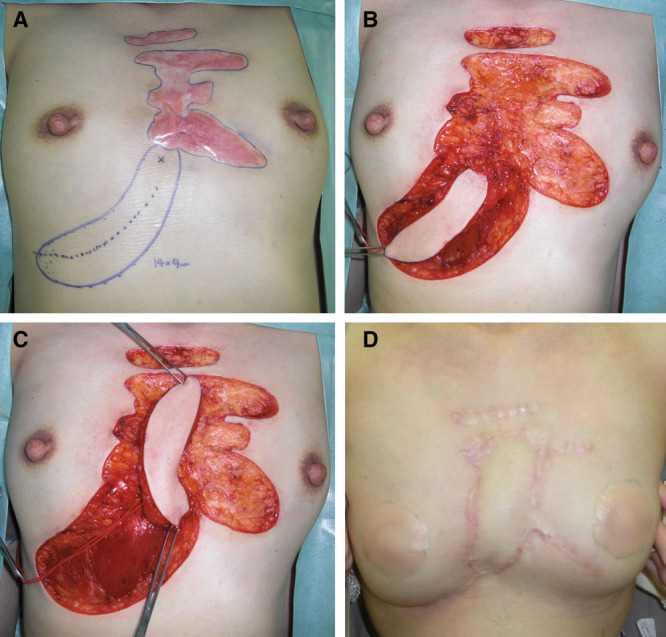
Case 3. This 43-year-old woman had had an anterior chest keloid since she was a teenager. She had received several steroid injections but stopped treatment by herself because of its relative ineffectiveness. In our department, we planned to remove all of her keloids and to replace the central part with a local perforator flap. The deep fascia of the rectus abdominis muscle was attached to the flap. This fascia was sutured to the fascia of the pectoralis major muscle on the recipient site. Three days after surgery, radiation therapy was started. The postoperative course was uneventful. A, Flap design. B, Keloid resection and flap elevation. C, Flap rotation. D, Postoperative view at 36 months.
Fig. 4.
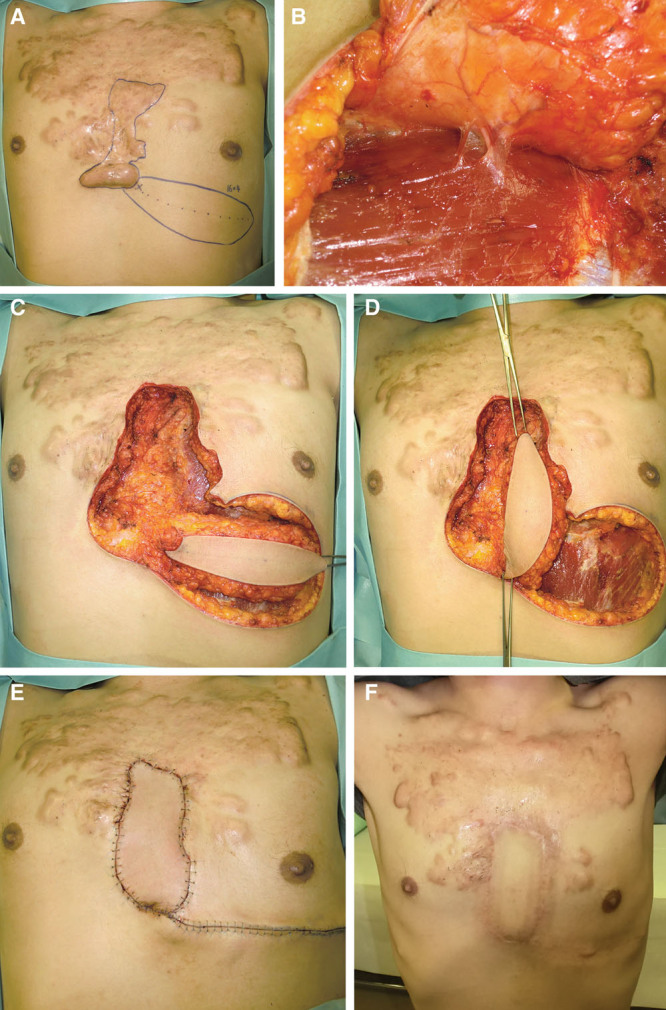
Case 7. This 39-year-old man had had an anterior chest keloid since he was a teenager. He had inclusion cysts on the central part of the chest wall that were getting bigger. The keloids were also getting bigger. In our department, we planned to remove the central part of the keloids only and to replace it with a local perforator flap. The deep fascia of the major pectoral muscle was attached to the flap. This fascia was sutured to the fascia on the recipient site. Three days after surgery, radiation therapy was started. The postoperative course was uneventful. A, Flap design. B, Seventh internal mammary artery perforator. C, Keloid resection and flap elevation. D, Flap rotation. E, Immediate postoperative view. F, Postoperative view at 24 months.
Fig. 3.
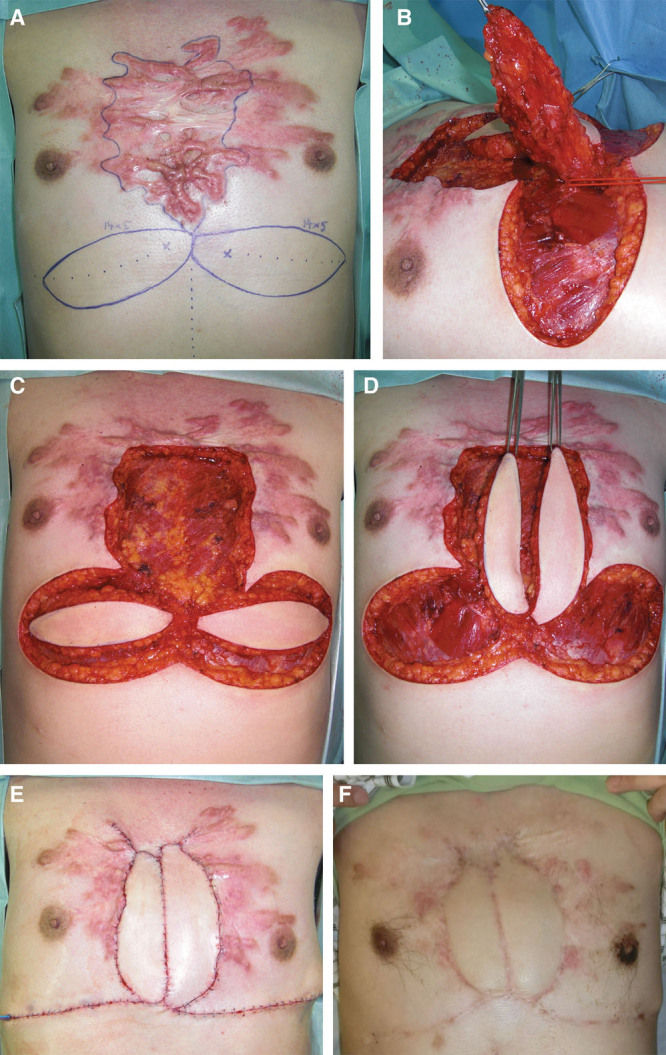
Case 4. This 46-year-old man had had an anterior chest keloid since he was a teenager. The central area contained inclusion cysts that had been infected several times and had each time been treated with incision followed by drainage. The keloids then started growing. In our department, we planned to remove the central part of the keloids only and to replace it with bilateral perforator propeller flaps. The deep fascia of the major pectoral muscle was attached to the flap. This fascia was sutured to the fascia on the recipient site. Three days after surgery, radiation therapy was started. The postoperative course was uneventful. A, Flap design. B, Flap elevation. C, Keloid resection and flap elevation. D, Flap rotation. E, Immediate postoperative view. F, Postoperative view at 32 months.
DISCUSSION
Factors That Drive Keloid Pathogenesis
Various genetic, systemic, and local factors influence the quality and quantity of keloids. A genome-wide association study5 and our research6 showed that four single-nucleotide polymorphism loci in three chromosomal regions associate significantly with the development of keloids in the Japanese population. There are probably many more genetic factors that drive keloid pathogenesis but have not yet been identified. In terms of systemic factors, adolescence and pregnancy seem to associate with a higher risk of developing keloids.8,9 It may be that sex hormones such as estrogens and androgens have vasodilatory effects that intensify inflammation and worsen keloids. Moreover, our recent study7 showed that hypertension associates with the development of severe keloids: hypertension may damage the blood vessels, thereby increasing the inflammation in scar tissue.
Although these risk factors are important, we believe that mechanical forces play a particularly significant role in the pathophysiology of keloids.1–4 Keloids commonly adopt distinct site-specific shapes (the typical butterfly, crab’s claw, or dumbbell shapes). These shapes seem to be largely determined by the direction of the tension applied to the skin around the wound site.4 Moreover, keloids show a marked preference for particular locations on the body: they usually occur at sites that are constantly or frequently subjected to tension (such as the anterior chest wall and scapular regions). By contrast, they seldom occur in areas where stretching/contraction of the skin is rare (such as the parietal region or anterior lower leg). This is true even for patients who have multiple/large keloids.
Although physicians cannot (or at least find it very difficult to) control genetic and systemic factors, they can reduce the mechanical forces around keloids. This can be achieved using various surgical techniques.10 In particular, we have found that skin flaps particularly effectively release tension.
Surgery and Postoperative Radiation Therapy for Keloids
It has been suggested that combining surgery with postoperative radiation therapy is more effective for keloids than radiation monotherapy15: the success rate of this combined approach varies between 67% and 98%.16 In many institutions, radiation is initiated right after surgery and the total dose, which is given over several treatments, is limited to 20 Gy. Moreover, Guix et al17 suggest that HDR-SB treatment for keloids is more effective than superficial X-ray or low-energy electron beam administration. Nevertheless, we have found that primary radiation therapy (ie, radiation monotherapy) is still useful for treating older patients or patients with severe acne-related keloids.10 The total radiation dose in these cases is higher than that used for postoperative radiation therapy. Therefore, it is necessary to apply the radiation carefully to prevent secondary radiation carcinogenesis. It is also important to obtain informed consent from the patient. We have found that radiation monotherapy immediately ameliorates subjective symptoms such as pain and itching and that the color and thickness of the scars normalize progressively over a year.
Notably, the present study has caused us to change our position regarding radiation therapy after partial resection/mass reduction of keloids. Initially, we believed that a patient should only be subjected to postoperative radiation if the keloids have been resected completely. Thus, in cases of partial resection, the radiation therapy was postponed until after the second round of surgery to remove the remaining keloid tissue. This was because we believed that the second round of surgery would also have to be followed by radiation therapy and that two radiotherapy sessions would expose the patient to too much radiation. However, the present study made us realize that after partial resection/mass reduction surgery and flap reconstruction, just one session of radiation therapy is sufficient for preventing recurrence (cases 4 and 7). Moreover, we found that even though the remaining keloids in these patients were not irradiated, the inflammation in the keloids adjacent to the flap decreased little by little over time after the surgery (Figs. 3, 4). These effects probably reflect the fact that the flap effectively reduces the tension around the remaining keloids, as will be discussed further below.
An important concern associated with keloid radiation therapy is the risk of inducing malignant tumors. However, when radiation oncologists in 508 facilities throughout the world were asked via a questionnaire about the indications of radiation therapy for keloids, more than 90% considered radiation therapy to be acceptable for keloids.18 Thus, the risk of carcinogenesis after radiation therapy may not be as high as is sometimes perceived by practitioners in other areas of medicine. Of course, it is necessary to follow up irradiated patients for as long as possible. It should also be noted that the risk has dropped recently because of the development of safer methods using appropriate dose and fractions.10,17–20 Thus, we recommend that plastic surgeons should not avoid radiation therapy; rather, they should discuss the possibility of using safer and more effective procedures with a radiation oncologist.
Flap Surgery for Keloids
Regardless of whether skin grafts or flaps are used for reconstruction after keloid resection, keloid recurrence can be controlled by delivering appropriate radiation therapy. However, skin grafts have a disadvantage relative to flaps; namely, they sometimes develop circular recurrence of the keloid from the edge of the grafted skin. This may reflect, at least in part, the fact that grafted skin cannot expand as much over time as flaps. This may cause the tension on the margin of the grafted skin to rise, thus generating new keloids. Moreover, in skin grafting, it is difficult to determine when to start radiation because grafted skin can have relatively weak vascularity in the early days of wound healing and this could be worsened by radiation therapy. By contrast, skin flaps are strongly vascular, even immediately after surgery, and thus they are less likely to be compromised by vascular damage caused by the radiation therapy. The development of propeller flaps has further increased this advantage of flaps over skin grafts. These flaps are harvested and then rotated around 90 degrees.21 This effectively releases the horizontal tension on the chest while increasing the vertical tension on the abdomen. Thus, this “tension reorganizing” approach transfers the original tension on the keloid elsewhere. This may explain why even in our cases of partial resection, propeller flap surgery was associated with the gradual amelioration of the remaining keloidal tissue.
In our experience with the IMAP flap, perforators can be readily found around the IMA. Thus, we believe that many chest wall keloids can be reconstructed by using the IMAP flap. Indeed, IMAP flaps have been used to repair median sternotomy wounds, chest wall fistula, and upper abdominal wounds.22–26 These studies confirm that the IMAP flap has stable vascularity and is useful. We believe that our method is a useful reconstruction method in cases of large keloids.
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Huang C, Murphy GF, Akaishi S, et al. Keloids and hypertrophic scars: update and future directions. Plast Reconstr Surg Glob Open. 2013;1:e25. doi: 10.1097/GOX.0b013e31829c4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogawa R, Okai K, Tokumura F, et al. The relationship between skin stretching/contraction and pathologic scarring: the important role of mechanical forces in keloid generation. Wound Repair Regen. 2012;20:149–157. doi: 10.1111/j.1524-475X.2012.00766.x. [DOI] [PubMed] [Google Scholar]
- 3.Ogawa R, Akaishi S, Huang C, et al. Clinical applications of basic research that shows reducing skin tension could prevent and treat abnormal scarring: the importance of fascial/subcutaneous tensile reduction sutures and flap surgery for keloid and hypertrophic scar reconstruction. J Nippon Med Sch. 2011;78:68–76. doi: 10.1272/jnms.78.68. [DOI] [PubMed] [Google Scholar]
- 4.Akaishi S, Akimoto M, Ogawa R, et al. The relationship between keloid growth pattern and stretching tension: visual analysis using the finite element method. Ann Plast Surg. 2008;60:445–451. doi: 10.1097/SAP.0b013e3181238dd7. [DOI] [PubMed] [Google Scholar]
- 5.Nakashima M, Chung S, Takahashi A, et al. A genome-wide association study identifies four susceptibility loci for keloid in the Japanese population. Nat Genet. 2010;42:768–771. doi: 10.1038/ng.645. [DOI] [PubMed] [Google Scholar]
- 6.Ogawa R, Watanabe A, Than Naing B, et al. Associations between keloid severity and single-nucleotide polymorphisms: importance of rs8032158 as a biomarker of keloid severity. J Invest Dermatol. 2014;134:2041–2043. doi: 10.1038/jid.2014.71. [DOI] [PubMed] [Google Scholar]
- 7.Arima J, Huang C, Rosner B, et al. Hypertension: a systemic key to understanding local keloid severity. Wound Repair Regen. 2015;23:213–221. doi: 10.1111/wrr.12277. [DOI] [PubMed] [Google Scholar]
- 8.Park TH, Chang CH. Keloid recurrence in pregnancy. Aesthetic Plast Surg. 2012;36:1271–1272. doi: 10.1007/s00266-012-9947-5. [DOI] [PubMed] [Google Scholar]
- 9.Ud-Din S, Volk SW, Bayat A. Regenerative healing, scar-free healing and scar formation across the species: current concepts and future perspectives. Exp Dermatol. 2014;23:615–619. doi: 10.1111/exd.12457. [DOI] [PubMed] [Google Scholar]
- 10.Ogawa R, Akaishi S, Kuribayashi S, et al. Keloids and hypertrophic scars can now be cured completely: recent progress in our understanding of the pathogenesis of keloids and hypertrophic scars and the most promising current therapeutic strategy. J Nippon Med Sch. 2016;83:46–53. doi: 10.1272/jnms.83.46. [DOI] [PubMed] [Google Scholar]
- 11.van Leeuwen MC, Stokmans SC, Bulstra AE, et al. Surgical excision with adjuvant irradiation for treatment of keloid scars: a systematic review. Plast Reconstr Surg Glob Open. 2015;3:e440. doi: 10.1097/GOX.0000000000000357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ayestaray B, Proske YM. Perineal and posterior vaginal wall reconstruction with a superior gluteal artery dual perforator-pedicled propeller flap. Microsurgery. 2015;35:64–67. doi: 10.1002/micr.22279. [DOI] [PubMed] [Google Scholar]
- 13.Gunnarsson GL, Jackson IT, Westvik TS, et al. The freestyle pedicle perforator flap: a new favorite for the reconstruction of moderate-sized defects of the torso and extremities. Eur J Plast Surg. 2015;38:31–36. doi: 10.1007/s00238-014-1043-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dumitrascu DI, Georgescu AV. Pedicled perforator flaps for covering the elbow region. A case report. Clujul Med. 2015;88:560–562. doi: 10.15386/cjmed-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Norris JE. Superficial x-ray therapy in keloid management: a retrospective study of 24 cases and literature review. Plast Reconstr Surg. 1995;95:1051–1055. doi: 10.1097/00006534-199505000-00015. [DOI] [PubMed] [Google Scholar]
- 16.Jones K, Fuller CD, Luh JY, et al. Case report and summary of literature: giant perineal keloids treated with post-excisional radiotherapy. BMC Dermatol. 2006;19:6. doi: 10.1186/1471-5945-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guix B, Henríquez I, Andrés A, et al. Treatment of keloids by high-dose-rate brachytherapy: a seven-year study. Int J Radiat Oncol Biol Phys. 2001;50:167–172. doi: 10.1016/s0360-3016(00)01563-7. [DOI] [PubMed] [Google Scholar]
- 18.Leer JW, van Houtte P, Davelaar J. Indications and treatment schedules for irradiation of benign diseases: a survey. Radiother Oncol. 1998;48:249–257. doi: 10.1016/s0167-8140(98)00051-6. [DOI] [PubMed] [Google Scholar]
- 19.Kuribayashi S, Miyashita T, Ozawa Y, et al. Post-keloidectomy irradiation using high-dose-rate superficial brachytherapy. J Radiat Res. 2011;52:365–368. doi: 10.1269/jrr.10159. [DOI] [PubMed] [Google Scholar]
- 20.Kim S, Choi TH, Liu W, et al. Update on scar management: guidelines for treating Asian patients. Plast Reconstr Surg. 2013;132:1580–1589. doi: 10.1097/PRS.0b013e3182a8070c. [DOI] [PubMed] [Google Scholar]
- 21.Pignatti M, Ogawa R, Hallock GG, et al. The “Tokyo” consensus on propeller flaps. Plast Reconstr Surg. 2011;127:716–722. doi: 10.1097/PRS.0b013e3181fed6b2. [DOI] [PubMed] [Google Scholar]
- 22.Schwabegger AH, Piza-Katzer H, Pauzenberger R, et al. The internal mammary artery perforator (IMAP) breast-flap harvested from an asymmetric hyperplastic breast for correction of a mild funnel chest deformity. Aesthetic Plast Surg. 2011;35:928–932. doi: 10.1007/s00266-011-9697-9. [DOI] [PubMed] [Google Scholar]
- 23.Shayan R, Syme DY, Grinsell D. The IMAP flap for pharygoesophageal reconstruction following stricture release. J Plast Reconstr Aesthet Surg. 2012;65:810–813. doi: 10.1016/j.bjps.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 24.Rüegg EM, Lantieri L, Marchac A. Dual perforator propeller internal mammary artery perforator (IMAP) flap for soft-tissue defect of the contralateral clavicular area. J Plast Reconstr Aesthet Surg. 2012;65:1414–1417. doi: 10.1016/j.bjps.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 25.Takeuchi M, Sakurai H. Internal mammary artery perforator flap for reconstruction of the chest wall. J Plast Surg Hand Surg. 2013;47:328–330. doi: 10.3109/2000656X.2012.718893. [DOI] [PubMed] [Google Scholar]
- 26.Koulaxouzidis G, Orhun A, Stavrakis T, et al. Second intercostal internal mammary artery perforator (IMAP) fasciocutaneous flap as an alternative choice for the treatment of deep sternal wound infections (DSWI). J Plast Reconstr Aesthet Surg. 2015;68:1262–1267. doi: 10.1016/j.bjps.2015.05.019. [DOI] [PubMed] [Google Scholar]


