Figure 2.
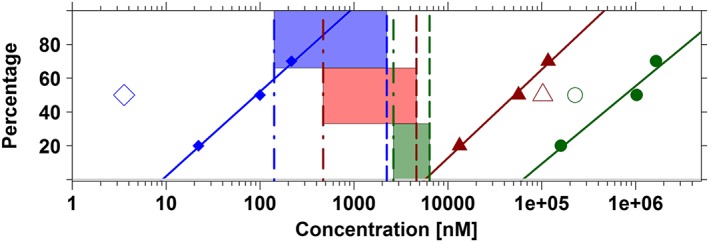
Lack of correlation between in vitro (dofetilide displacement, functional assay) and in vivo (probabilities of QT prolongation in vivo in dogs and humans) data. Ideally, proportional differences should be found between these experiments, reflecting the relative differences in the potency of the different compounds. Data summaries include cisapride (blue diamonds), sotalol (red triangles) and moxifloxacin (dark green dots). Log‐linear regression (solid lines) of the estimates of IC20, IC50 and IC70 obtained from the dofetilide displacement are compared with the IC50 values obtained from hERG patch clamp assays (open symbols) and in vivo (dashed vertical lines) and clinical (dot‐dashed vertical lines) concentrations corresponding to 50% probability of QTc prolongation ≥10 ms (i.e. CP50). The window associated with these concentrations (i.e. CP50) in dogs and humans is depicted using the blue, red and dark green shaded areas for cisapride, sotalol and moxifloxacin, respectively. For instance, for cisapride, IC50 in the binding assay indicates a potency of 99.9 nM. In contrast, potency in the hERG functional assay is much higher, that is, 3.57 nM. These values contrast with CP50 estimate in vivo in dogs and in healthy subjects, which yield estimates of 2233.6 and 141.5 nM, respectively. See Table 5 for further details. Parameters describing the log‐linear regression for cisapride, sotalol and moxifloxacin were respectively y = −49.85 + 49.91*log(x), y = −196.08 + 52.22*log(x) and y = −220.46 + 45.96*log(x).
