Abstract
In migratory species, breeding and non-breeding locations are geographically separate, yet the effects of conditions from one stage may carry over to affect a subsequent stage. Ideally, to understand the mechanisms and implications of ‘carry-over effects’, one would need to follow individuals throughout the year, quantify potential environmental causal factors and physiological mediators during multiple life-history stages, and measure downstream fitness. Owing to current limitations of tracking technology, this is impossible for small, long-distance migrants, so indirect methods to characterize carry-over effects are required. Corticosterone (CORT) is a suspected physiological mediator of carry-over effects, but when collected from blood it provides only a physiological snapshot at that point in time. When extracted from feathers, however, feather corticosterone (CORTf) provides a measure of responses to stressors from previous, and longer, time periods. We collected feathers grown during two life-history stages (post-breeding and subsequent wintering) from individuals of two age classes of a rapidly declining migratory songbird, the cerulean warbler (Setophaga cerulea), on their breeding grounds and quantified CORTf concentrations. We then monitored reproduction and survival of individuals and analysed relationships among CORTf and age, body condition and future fitness. Compared with older males, second-year males had higher CORTf concentrations during both stages. When controlling for age and year, body condition at capture was positively related to CORTf concentrations from winter (especially for older birds). However, we found no relationships between CORTf and fitness (as defined by reproduction and survival). Thus, elevated CORT may represent a beneficial physiological response (e.g. hyperphagia prior to migration), particularly for certain life-history stages, and may mediate the condition in which individuals transition between stages. But for those birds that survive migration, subsequent fitness is likely determined by more recent events and local conditions (i.e. on breeding grounds), which have the potential to counteract conditions from the winter.
Keywords: Carry-over effects, cerulean warbler, hormone biomarker, Neotropical–Nearctic migrant, non-breeding, Setophaga cerulea
Introduction
Animals face a variety of environmental challenges during different stages of their annual cycles. In migratory species, breeding and non-breeding locations are geographically separate, yet despite this, the effects of conditions experienced during one stage can carry over to affect performance during a subsequent stage (Norris and Marra, 2007; Harrison et al., 2011; Tonra et al., 2014). In order to gain a complete understanding of the mechanisms and implications of these ‘carry-over effects’, researchers need to follow individuals throughout the year and quantify potential environmental causal factors (e.g. food availability, pollutants) and physiological mediators (e.g. body condition, stress physiology) during multiple life-history stages, as well as measure downstream fitness effects themselves (i.e. future reproduction and survival). Carry-over effects have been directly quantified for some larger resident or short-distance migrants (e.g. Salton et al., 2015), but this is virtually impossible for many species, particularly small migratory birds that make rapid, long-distance migrations and cannot be monitored on a daily basis throughout their entire life cycle (e.g. Stutchbury et al., 2009; DeLuca et al., 2015). As a result of this, few studies have been able to relate physiological correlates of environmental conditions faced by small migratory birds from multiple life-history stages to subsequent components of fitness at the individual level. This is an important shortcoming in our understanding of how populations are limited, because many species of small migratory songbirds increasingly face human-caused threats throughout the annual cycle (Webster and Marra, 2005), yet most research has focused only on the breeding period (Marra et al., 2015).
The main proposed mechanism of carry-over effects is non-lethal variation in physiological ‘state’ attributable to environmental factors (Harrison et al., 2011), such as diet (Sorensen et al., 2009; Kouwenberg et al., 2013), especially in low-quality habitats (Marra et al., 1998). As it is often difficult to measure physiological state directly, short-term measures, such as body condition indices and blood concentrations of the hormone corticosterone (CORT), have been used as indirect measures. Corticosterone is especially relevant to carry-over effects because it is integral to energy management and is released in quantity upon exposure to challenging or threatening environmental conditions (i.e. stressors), such as low food availability, predators, anthropogenic disturbances or poor weather (i.e. the purported drivers of carry-over effects; Wingfield et al., 1983; Marra and Holberton, 1998; Wingfield and Kitaysky, 2002; Kitaysky et al., 2010; Harrison et al., 2011; and see Fairhurst et al., 2015a). By orchestrating changes in physiology and behaviour aimed at maximizing survival, CORT provides a hypothesized functional link between environmental variation and fitness.
Corticosterone-mediated responses to environmental conditions during the non-breeding stage may underlie carry-over effects to the breeding stage in migratory birds. Limited previous work supports this hypothesis. For example, American redstarts (Setophaga ruticilla) that wintered in higher-quality habitats in Jamaica had, on average, lower levels of baseline CORT (Marra and Holberton, 1998), departed from wintering grounds and arrived on breeding grounds earlier and in better condition (Marra et al., 1998), fledged more young (Norris et al., 2004) and returned to wintering grounds at greater rates (Studds and Marra, 2005). Likewise, in Arctic-breeding common eiders (Somateria mollissima), birds with higher CORT during the non-breeding stages arrived later on the breeding grounds and were in poorer body condition, which led to poorer reproductive performance (Harms et al., 2015). In contrast, however, Atlantic Puffins (Fratercula arctica) with elevated CORT during the non-breeding stage subsequently laid larger eggs (Kouwenberg et al., 2013), which is typically related positively to fitness (Krist, 2011). These studies, with their contrasting results, suggest that: (i) CORT during the non-breeding stage can mediate carry-over effects into the breeding stage; but (ii) the directionality of the relationship between CORT and future performance traits may be context dependent. Moreover, physiological responses to stressors may co-vary with other factors that play important roles in future survival or reproduction. For example, younger individuals may struggle to locate food because they may be naïve to their non-breeding environment (Wunderle, 1991; Daunt et al., 2007) and thus may elevate CORT concentrations to increase survival (Lendvai et al., 2015), but they may fail to reproduce successfully because of lack of experience, regardless of their level of stress (Martin, 1995; Hawkins et al., 2012). However, to our knowledge no previous study has assessed individual-level relationships between CORT from non-breeding and subsequent reproduction in multiple age groups, so our understanding of the role of age in influencing carry-over effects remains limited.
In this study, we hypothesized that CORT during non-breeding mediates carry-over effects to the breeding season and that this relationship is age dependent. We tested this in a long-distance avian migrant, the cerulean warbler (Setophaga cerulea), a songbird with severely declining populations in North America (Buehler et al., 2013, Sauer et al., 2014). By analysing CORT from tail and rump feathers (hereafter, CORTf) collected from males on the breeding grounds, we retrospectively quantified physiology from two life stages: the prior non-breeding stage (rump) and the post-breeding/post-fledgling stage that preceded it (tail). This allowed us to determine whether there was individual consistency in CORTf during two stages of the annual cycle leading up to reproduction. We then related CORTf from these previous stages to body condition, reproduction and apparent survival of the same individuals collected subsequently on the breeding grounds, while considering two potentially confounding factors of both CORT and reproduction (age and year).
We tested three predictions related to our hypothesis. First, compared with older (after-second-year; ASY) males, we expected younger (second-year; SY) males to have greater CORTf in both life-history stages (post-breeding/post-fledgling and non-breeding) and, subsequently, poorer body condition on breeding grounds, reflecting a lack of dominance and experience and, potentially, the use of lower-quality habitat. Second, we expected a relationship between CORTf during non-breeding and subsequent body condition during breeding in both age classes, but recognize that the direction of this relationship may be context dependent (e.g. age dependent). Experimentally testing the mechanism(s) underlying such an association would be logistically impossible, so we explored this relationship correlatively, predicting that the relationship would be negative if poor condition during non-breeding and/or use of poorer-quality non-breeding habitats led to physiological and/or behavioural costs (e.g. poor condition prior to spring migration and/or delayed spring migration) associated with elevated CORT. In contrast, we would expect a positive relationship if good condition and/or use of good-quality non-breeding habitats allow birds to benefit from the behavioural effects or physiological results of elevated CORT during that time (e.g. increased hyperphagia and improved pre-migratory condition and/or earlier departure). Third, we expected CORTf to correlate with one or more fitness parameters, but this could also be context dependent. If higher concentrations of CORT reflect poor body condition, then males with higher CORTf may be paired less frequently, initiate nesting later, be less likely to breed successfully, produce fewer young and/or be less likely to return. Alternatively, if higher CORT reflects individuals in better condition, then males with higher CORTf would be more likely to pair, initiate nesting earlier, breed successfully, produce more young and/or be more likely to return the following year.
Materials and methods
Study species
Cerulean warblers are small Nearctic–Neotropical migratory birds that nest in the canopy of mature deciduous forests of eastern USA (Buehler et al., 2013) and are typically socially monogamous (but see Boves and Buehler, 2012), obligately single brooded, and will attempt to re-nest up to two times after failure (Buehler et al., 2013). On their wintering grounds in the northern Andes of South America (from north Colombia and Venezuela to northwest Bolivia >500 m above sea level), they use a variety of habitats, including primary and secondary forest (Buehler et al., 2013) as well as agroforestry plantations (Bakermans et al., 2009). Over the past 50 years, the global population of cerulean warblers has declined >3%/year (Sauer et al., 2014), and they are considered vulnerable, or a species of conservation concern, by many agencies and organizations (Buehler et al., 2013). The cause of this precipitous decline is not entirely clear, but a number of broad factors have been proposed, including a decrease in habitat quality on the breeding grounds (Boves et al., 2013) and a decrease in habitat quantity and quality on the wintering grounds (Buehler et al., 2013).
Moult location in cerulean warblers
Although only minimal direct evidence of moult location in cerulean warblers exists (for either tail or rump), and we cannot be certain that every feather that we analysed was grown in the location we assume, the most likely pattern of moult (for individuals from both age classes) is that: (i) rectrices are moulted on the post-breeding grounds (or, less likely, during migration), and (i) rump feathers are moulted on their Andean wintering grounds. We use evidence from stable isotopes, plumage colouration, feather wear and migration timing to make these conclusions and describe each below.
After-second-year rectrices
Analysis of hydrogen stable isotopes suggests that tail feathers for ASY birds are grown on the breeding grounds or, for a small proportion of these older birds, early during migration (before leaving the USA; C.S.R., unpubublished data). In our estimation, moult-migration is more unlikely because of the high energetic cost of both of these activities and because cerulean warblers appear to be both early (often leaving the USA in early August) and rapid migrants (T.J.B., unpubublished geolocator data, personal observation).
Second-year rectrices
Juvenile cerulean warblers grow tail feathers that resemble SY males by 30 days post-fledging. Upon returning to the breeding grounds, tail feather wear is substantial, suggesting that these feathers have not been moulted more recently, i.e. on the wintering grounds (T.J.B., personal observation; D. W. Raybuck, personal communication, unpubublished data).
Second-year rump
Shortly after fledging, and into their first migration, juvenile and hatch-year (HY) male cerulean warblers possess body plumage that is different in colour from definitive adult male plumage (greyer and greener, similar to female; Pyle, 1997). The rump feathers we analysed, from both SY and ASY birds, were much bluer than this juvenile male plumage (Boves et al., 2014), suggesting a wintering grounds origin of the rump feathers. Hydrogen stable isotopes also support the suggestion that these feathers are grown on wintering grounds or during migration (C.S.R., unpubublished stable isotope analysis data). Moult-migration is more unlikely because of the same limitations described above.
After-second-year rump
Hydrogen stable isotope analysis suggests that these feathers are grown either on wintering grounds or during migration (C.S.R., unpublished data). Moult-migration is more unlikely because of the same limitations described above. No plumage or feather wear characteristics are useful in identifying the moult location for these feathers from ASY birds, but body moult has been documented during the winter in this age class (F. Newell, personal communication) and, to our knowledge, has not been observed during post-breeding or migration.
Study area
We studied cerulean warblers from 2008 to 2010 on ~160 ha in the North Cumberland Wildlife Management Area, Campbell County, TN, USA (36°12′N, 84°16′W and 36°21′N, 84°18′W). Cerulean warblers are found at some of their greatest breeding densities in this region (Buehler et al., 2006); mean density of cerulean warblers ranged from 0.67 to 0.74 territories/ha/year (T.J.B., unpubublished data). The forest type was mostly mixed mesophytic, and tree composition was predominantly oaks (Quercus spp.), maples (Acer spp.), hickories (Carya spp.) and tulip poplar (Liriodendron tulipifera).
Field methods
Capture, feather collection and morphometrics
We captured male cerulean warblers during the breeding season, from early May to mid-June. To capture individuals, we broadcast territorial songs and call notes and displayed a male conspecific decoy strategically set near a mist net. After capture, we aged birds as second-year (SY; i.e. in their first breeding season) or after-second-year (ASY; i.e. in or after their second breeding season) on the basis of moult limits and feather wear. Like many warblers species, SY male cerulean warblers retain some brownish juvenile alular feathers and primary coverts, and possess worn and pointed rectrices (Pyle, 1997). Once captured, we banded each bird with a unique combination of a federally issued aluminum leg band and one to three plastic coloured leg bands to enable later identification of individuals in the field without recapture; we also collected five rump feathers and one tail feather (right innermost rectrix). Finally, we measured right wing chord to the nearest 0.5 mm (using a straight wing rule) and mass to the nearest 0.1 g (using a digital scale).
Pairing, nest searching and monitoring
For all birds, we searched each territory for a minimum of 4 h/week during the entire breeding season (from May to early July) to determine pairing status and to search for nests. In addition, we conducted intensive spot-mapping sessions on eight mornings during the peak of the breeding season. We considered a male to be paired in the following circumstances: (i) if a nest was constructed within the territory of the male; (ii) if no nest was located, but a female was observed repeatedly within the male's territory; or (iii) if we observed male behaviour that would strongly suggest pairing (e.g. male carrying nesting material or food items to young). Despite identifying these criteria for pairing a priori, we successfully located nests for every paired individual in our sample and thus did not ever use criteria (ii) or (iii) to determine pairing. We used behavioural cues to find nests (e.g. nest material collection, building, foraging/provisioning), and we found the majority of nests (>75%) during the building stage. After a nest was located, we monitored it every 1–3 days to determine its fate. If nestlings survived to 7 days of age, we monitored nests daily for ~45 min with a spotting scope equipped with a ×60 eyepiece. We then counted nestlings daily (this was most effective when parents came to feed) until fledging, and on the day of fledging, we searched for fledglings in the area surrounding the nest to confirm success or failure. If a nest was successful, we considered the number of nestlings on the last day in the nest (typically day 9 or 10) to be the number of young fledged. Between days 7 and 10 of the nestling stage, we also video-recorded nests for 2 h (between 06.30 and 10.00 h) to confirm nestling counts and estimated male provisioning rates per nestling/h; we did not record nests on mornings with high winds or precipitation. When a nest failed, we returned to the territory and searched for evidence of re-nesting in the same manner used to find initial nests. Re-nesting attempts were often easier to locate because of the increased rate of building and the re-use of previous nesting material. We continued this process for all territories until the end of the breeding season (mid-July).
Annual survival
To assess future (apparent) survival, we returned to sites in subsequent years to resight colour-marked males across all the 160 ha study area and in adjacent areas ≤200 m of study area boundaries. In >8 years of studying this species in this area, nearly all (98%) known returning birds have settled within 100 m of previous season territorial boundaries (T.J.B. and D.A.B., unpubublished data), so we believe the 200 m buffer was sufficient to resight most individuals that returned to the general vicinity.
Estimates of body condition
We estimated body condition at capture from simple body mass measurements (using a digital scale). Although body mass can vary over time, we have found that during the breeding season there is little change in mass within individuals (T.J.B., unpublished data), but we did account for date of capture by including it as a random effect in our models. Body mass is often as or more reliable than unverified indices (Schamber et al., 2009), but we also evaluated an index of body condition by regressing mass on wing length and used the resulting residuals (Schulte-Hostedde et al., 2005).
Feather corticosterone analysis
Extraction of CORT from feathers followed Bortolotti et al. (2008), which has been replicated with a variety of passerine species (Fairhurst et al., 2014, 2015b; Grunst et al., 2015). Given that we collected multiple rump feathers from each bird, we analysed two rump feathers per individual to ensure that hormone concentrations were detectable. Only a single rectrix was analysed for each bird because we collected only one per individual. We measured the length of each feather and, for rectrices, we removed the calamus before extraction; for the much smaller rump feathers, the calamus was minute, and proper removal (i.e. distal to the superior umbilicus) was not possible, so we included it in the samples. We cut each sample into pieces <5 mm2 with scissors, added 10 ml of methanol (HPLC grade; Fisher Scientific, Fairlawn, NJ, USA), and placed the samples in a sonicating water bath at room temperature for 30 min, followed by incubation at 50°C overnight in a water bath. We separated the methanol extract from feather material by vacuum filtration, using a plug of synthetic polyester fibre fitted in a filtration funnel. We then placed all methanol extracts in a 50°C water bath and evaporated them in a fume hood. We reconstituted extract residues in a small volume of phosphate-buffered saline (0.05 M, pH 7.6), transferred them to Eppendorf tubes, and froze them at −20°C. We assessed the efficiency of the methanol extraction by spiking three feather samples with a small amount (~5000 counts/min) of 3H-corticosterone in the extraction. We recovered 97.9% of the radioactivity in the reconstituted samples. All samples were extracted in a single batch.
The CORTf concentrations were determined by radioimmunoassay following Wayland et al. (2002) and used a commercially available antibody (Sigma-Aldrich, St Louis, MO, USA; catalogue no. C8784). Measurements were performed in duplicate on reconstituted methanol extracts, and serial dilutions of samples were parallel to the CORT standard curve. Samples were measured in two assays, and the variability of each assay was assessed using six replicates of an internal standard. The average intra-assay coefficient of variation was 9.0% (SD = 2.1), and inter-assay coefficient of variation was 5.8%. The assays had an average detectability limit (%B/B0) of 21.1 pg CORT (SD = 4.8), but all samples were well above detectability limits. Data values are expressed as picograms of CORT per millimetre of feather, which gives a valid estimate of CORT per unit time of feather growth (Bortolotti et al., 2008; Bortolotti, 2010). Resulting CORTf concentrations, for both feather types, were not correlated with sample feather mass within either age class (rump ASY, F = 0.36, d.f. = 1,37, P = 0.55; rump SY, F = 0.76, d.f. = 1,19, P = 0.39; rectrix ASY, F = 0.59, d.f. = 1,40, P = 0.45; and rectrix SY: F = 0.79, d.f. = 1,17, P = 0.39), suggesting that any differences in CORTf concentrations between age classes were not attributable to the potential influence of feather mass. The CORT assays were performed at the University of Saskatchewan, Canada.
Statistical analyses
Recent data suggest that CORTf values are similar between different feather types (Lendvai et al., 2013), feathers grown on opposite sides of the bird (e.g. left and right wing), and adjacent feathers of the same type (see Romero and Fairhurst, 2016 for a review). However, given that the rump and rectrix feathers in our study were grown at different places and in different seasons (see ‘Moult location in cerulean warblers’ above), we did not expect these different feather types to reflect similar concentrations of plasma CORT. Thus, we avoided making direct comparisons between rump and rectrix CORTf values in our study, and conducted separate statistical analyses for each feather type.
Relationship between feather corticosterone, age and year
We assessed the relationship of age and year with CORTf by constructing general linear models, with CORTf concentration as the response variable, and age, year and age × year as predictor variables. We examined distributional plots of univariate variables as well as residuals and tested for violations of assumptions of normality (Shapiro–Wilk W test) and constant variance (Levene's test). We found no violations for rump feathers, but for rectrices the data were positively skewed. We corrected the skewness by a reciprocal transformation of rectrix CORTf, which was applied to all subsequent models using this variable. For both feather types, if the interaction term was not significant (P > 0.05), we removed this term and re-ran the simplified models.
To determine whether there was individual consistency in CORTf values between seasons, we assessed the relationship between rump CORTf and rectrix CORTf using a general linear mixed model with bird identity included as a random effect. This model used rump CORTf as the response variable (because it was grown after the rectrix) and included an effect of age and a rectrix CORTf × age interaction term as predictor variables. We were not interested in annual differences in this relationship and instead wanted to know whether there was a general relationship across all years, so we included year as a random term in this model. We included individual bird identity as a random term to account for the fact that we measured rump CORTf and rectrix CORTf in the same individuals.
Relationship between feather corticosterone and body condition metrics
We evaluated potential carry-over effects of CORT on two response variables related to body condition, namely body mass and a body condition index derived from body mass–wing length residuals. Each response variable was modelled separately with a general linear mixed model and included the predictors of rump CORTf, age, and a rump CORTf × age interaction term. Year was included as a random effect in the model because we were not interested in annual differences but instead were concerned with whether the relationship between body condition and rump CORTf was a general effect across years. Date of capture was also included as a random effect in the model because birds were captured through the breeding season, and we wanted to generalize our results across all potential capture dates. After first running saturated models, we removed non-significant interaction terms (P > 0.05) and re-ran the models in more simplified forms.
Relationship between feather corticosterone and fitness parameters
We evaluated the relationship between non-breeding CORT and future reproduction and survival of individuals by testing the relationship between rump CORTf and several response variables related to fitness: first egg date (running date, May 1 = 1), number of fledglings produced (over entire season), pairing success (yes/no; Y/N), nesting success (Y/N), provisioning rate (no. of visits/h/nestling) and apparent annual survival (Y/N). For each response variable, we constructed generalized linear mixed models, which included the predictors of rump CORTf, age, and a CORTf × age interaction. Year was included as a random effect in all models except for annual survival, where the model would not converge with mixed effects, so we included year as a fixed effect. We examined residuals and tested for violations of assumptions appropriate to the type of model used (normal or binomial distribution). To meet assumptions of normality and constant variance of residuals, we logarithmically transformed first egg date and the number of fledglings produced (after adding a constant of 0.5 to each value to allow logarithmic transformation of zeros). After first running saturated models, we removed non-significant interaction terms (P > 0.05) and re-ran the models in more simplified forms. Finally, to ensure that habitat conditions did not confound results, we also included average territory basal area (in square metres per hectare) to control for the potential effects of breeding habitat variability on reproductive measures. If inclusion of basal area had no impact on the statistical relationship between CORTf and fitness measures (i.e. did not change the statistical significance in either direction), we removed this variable from the models. All statistics presented were derived from final models. Sample sizes vary by response variable for two reasons: (i) we censored some individuals if their inclusion in the analysis was illogical (e.g. we did not include unpaired individuals or those whose nests failed earlier in provisioning analysis); and (ii) we were uncertain about some information for individuals (e.g. unsure whether an individual reproduced successfully). All statistical analyses were conducted using SAS or JMP (SAS Institute, Cary, NC, USA). Untransformed means are presented ± 1 SEM.
Results
Relationship between feather corticosterone, age and year
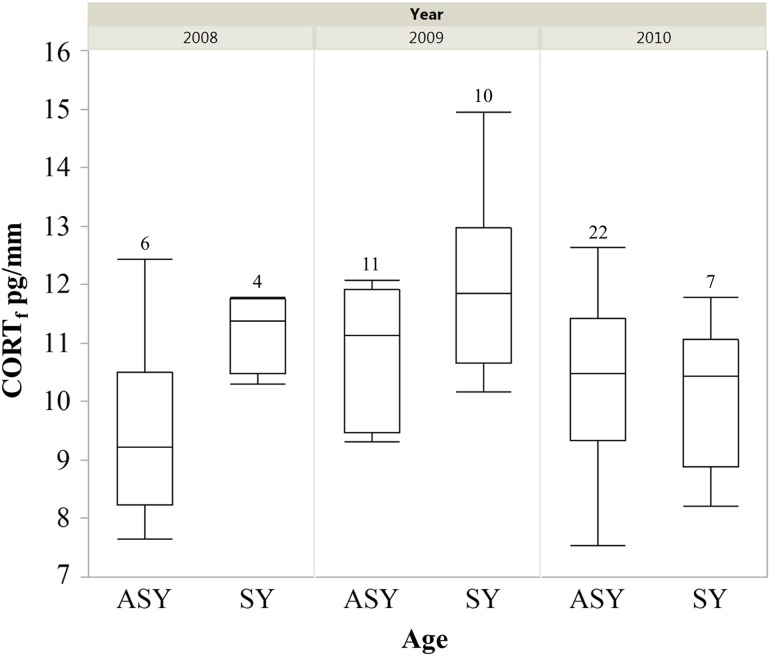
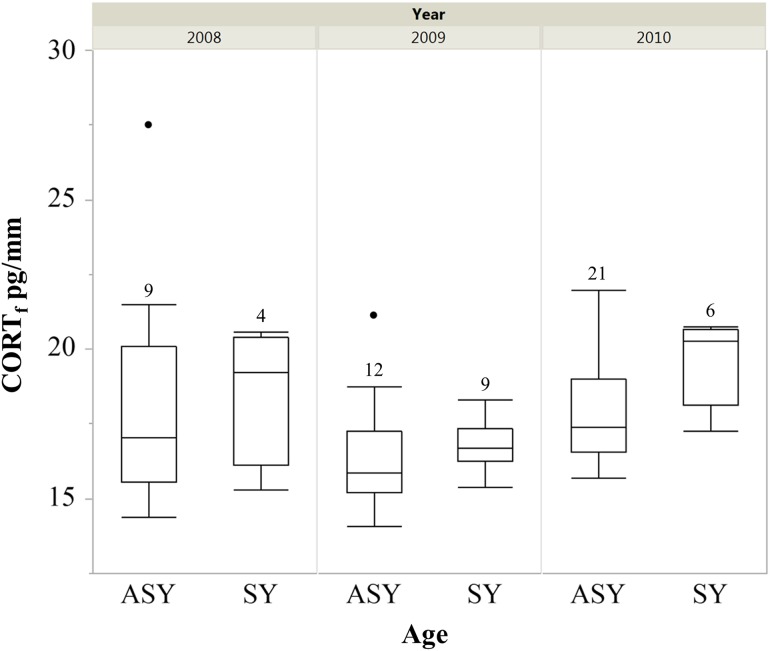
For rump feathers (grown on wintering grounds), young (SY) male cerulean warblers had greater CORTf values than older (ASY) males when controlling for year (SY, n = 21, = 11.26 ± 0.35 pg/mm; and ASY, n = 39, = 10.34 ± 0.21 pg/mm; χ2 = 5.83, d.f. = 1,54; P = 0.02; Fig. 1). The CORTf concentrations also varied annually when controlling for age (χ2 = 9.96, d.f. = 2,54, P = 0.01; Fig. 1) and the interaction of age × year was also significant (χ2 = 5.94, d.f. = 2,54, P = 0.05); however, this interaction appears minimal (see Fig. 1). For rectrix feathers (grown on post-breeding grounds), SY males had greater CORTf concentrations (reciprocally transformed) than ASY males (SY, n = 19, = 18.04 ± 0.43 pg/mm; and ASY, n = 42, =17.46 ± 0.37 pg/mm; χ2 = 3.81, d.f. = 1,57, P = 0.05; Fig 2). CORTf also differed by year when controlling for age (χ2 = 10.98, d.f. = 2,57, P = 0.004; Fig 2), but the interaction was not significant (χ2 = 1.28, d.f. = 2,55, P = 0.52) and was removed from the final model.
Figure 1:
Box plots displaying concentrations of corticosterone in rump feathers (CORTf) of male cerulean warblers from two age classes collected from 2008–10 in the Cumberland Mountains, TN, USA. The CORTf concentration differed between age classes when controlling for year (χ2 = 5.83, d.f. = 1,54, P = 0.02). ASY, after-second-year; SY, second-year.
Figure 2:
Box plots displaying concentrations of corticosterone from rectrix feathers (CORTf) of male cerulean warblers from two age classes collected from 2008–10 in the Cumberland Mountains, TN, USA. The CORTf concentration differed between age classes when controlling for year (χ2 = 3.81, d.f. = 1,57, P = 0.05). ASY, after-second-year; SY, second-year. Sample size is indicated above the bars.
We failed to find individual consistency between CORTf in rump CORTf and rectrix CORTf (F = 2.53, d.f. = 1,15.8, P = 0.13). The rectrix CORTf × age interaction was not significant (F1,49 = 2.38, P = 0.13) and was removed from the final model.
Relationship between non-breeding feather corticosterone and body condition
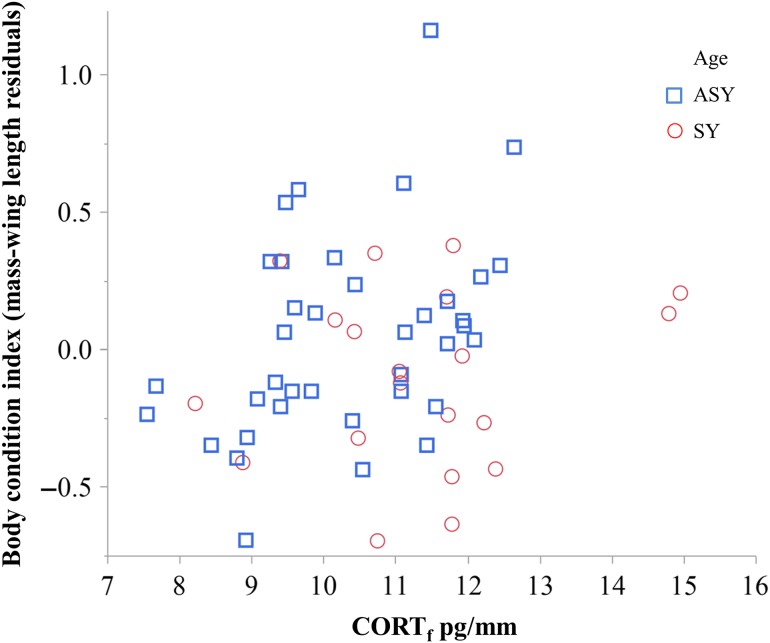
Body mass at capture was positively related to rump CORTf (F = 4.21, d.f. = 1,53.9, P < 0.05), after controlling for the effect of age (ASY males were heavier than SY males; F = 14.83, d.f. = 1,52.8, P = 0.0003). Likewise, body mass residuals were positively related to rump CORTf (F = 5.61, d.f. = 1,54.9, P = 0.02; Fig. 3), after controlling for the effect of age (F = 3.47, d.f. = 1,54.1, P = 0.07). The rump CORTf × age interaction term was not significant in either model (both P > 0.41) and was removed from both final models.
Figure 3:
The concentration of corticosterone in male cerulean warbler rump feathers (CORTf) grown during non-breeding was positively related to body condition while controlling for age and year (F = 5.61, d.f. = 1,54.9, P = 0.02) during the subsequent breeding season in the Cumberland Mountains, TN, USA, during 2008–10. ASY, after-second-year; SY, second-year.
Relationship between non-breeding feather corticosterone and fitness parameters
We found no relationship between rump CORTf and any of the reproductive or survival metrics (while controlling for age and year; Table 1). All CORTf × age interaction terms were non-significant (all P > 0.24), and territory basal area had no effect on inference for any variable (all P > 0.56), so both terms were removed from all final models.
Table 1:
Results from general linear models evaluating the relationship between predictor variable of rump corticosterone and various body condition and fitness-related response variables in male cerulean warblers (Setophaga cerulea) captured in the Cumberland Mountains, TN, USA, 2008–10
| Response variable | n | β estimate | F-value | P-value |
|---|---|---|---|---|
| Body condition index | 59 | 0.07 | 5.61 | 0.02* |
| First egg date | 43 | −0.03 | 1.65 | 0.21 |
| No. of fledglings produced | 49 | −0.004 | 0.01 | 0.94 |
| Pairing status (Y/N) | 58 | −0.18 | 0.74 | 0.39 |
| Nest success (Y/N) | 54 | 0.02 | 0.01 | 0.91 |
| Provisioning rate (visits/h per nestling) | 23 | −0.10 | 0.42 | 0.52 |
| Apparent annual survival (Y/N) | 60 | 0.18 | 0.65 | 0.42 |
All models controlled for age and year (random). Body condition index also controlled for date of capture (random; see Materials and methods). An * indicates statistical significance.
Discussion
Non-lethal carry-over effects from previous seasons and geographical locations have recently been suggested as important contributors to individual fitness differences and population declines in species that undergo seasonal migrations (Norris et al., 2004; Norris and Marra, 2007; Harrison et al., 2011). Corticosterone has been implicated as a physiological mediator of these seasonal interactions (Fairhurst et al., 2015b; Harms et al., 2015), and our study explored this idea using feathers grown during two different life-history stages (post-breeding moult and wintering moult) in younger (SY) and older (ASY) male cerulean warblers. In general agreement with our first two predictions, we found that SY birds had overall higher CORTf concentrations than ASY individuals at both moult stages (and there was no individual consistency in CORTf from post-breeding to wintering), and CORTf was related (positively) to body condition during the subsequent breeding season, and this relationship appeared to be stronger in ASY birds. Despite these relationships, individual CORTf concentrations were not related to any measure of future fitness (contrary to our third prediction). These results suggest that these birds may have benefitted from elevated CORT levels (e.g. via hyperphagia), but this depends on life-history stage, and these short-term benefits may not necessarily affect overall reproduction and survival. We discuss the implications of each of these results below.
Age and year
Our results provide important age-specific data on CORTf in a Neotropical migrant songbird. Young birds are naïve to many of the challenges that they face during their first year of life. Thus, birds undergoing their first migration and wintering in unfamiliar locations may be relegated to lower-quality wintering habitats and may not be able to cope with adverse environmental conditions as well as experienced adult birds. Accordingly, younger cerulean warblers had higher concentrations of CORTf than older birds during both moulting periods and in most years. These results are consistent with the constraints associated with the first year of life for migratory songbirds (e.g. McKim-Louder et al., 2013), particularly during migration, when the greatest mortality rates are believed to occur (Sillett and Holmes, 2002; Klaassen et al., 2014).
The age-based pattern of CORTf levels could also be explained by life-history theory, whereby older birds (that have fewer opportunities for future reproduction) may suppress their stress response to ensure no hormonal interference with, and conserve energy for, their limited remaining opportunities for reproduction (Wingfield and Sapolsky, 2003; Lendvai et al., 2015). However, even though young birds displayed higher concentrations of CORTf overall, this relationship varied somewhat by year (particularly for feathers grown on the wintering grounds). This demonstrates flexibility in CORT physiology and suggests that local conditions on wintering grounds determine CORT responses as much as, or even more so, than life-history stage. Individual-based differences in CORT physiology would also fail to explain the relationship between age and CORTf, because CORT concentrations in feathers grown at different times of the year were not correlated within birds. We would expect CORT concentrations in feathers from the same individual grown in different environmental conditions to be correlated if they were largely determined by endogenous (rather than environmental) factors. Sampling the same birds in multiple seasons and over multiple years would be necessary to show a true lack of consistency, but our results suggest individual flexibility from year to year and between life-history stages within a year (similar to Ouyang et al., 2011). In addition, because cerulean warblers are migratory and the CORT physiology that we measured preceded breeding by several months, a life-history strategy (related to age and reproduction) explaining the age-based pattern of CORT seems unlikely.
Annual fluctuations in weather (and associated food availability) could explain the annual variation in CORTf that we detected. Of particular importance to cerulean warblers on the wintering grounds may be the El Niño–Southern Oscillation phenomenon, which can have profound effects on weather across the Andes (Garreaud 2009). During the cold ocean phases (La Niña), air temperatures are depressed and precipitation is increased in the northern Andes. If in place for long time periods, these conditions may lead to decreased insect food availability (relative to short-term La Niña or El Niño events; Van Bael et al., 2004). We recorded relatively high levels of CORTf from rump feathers of birds captured in 2009, which followed 2 years of La Niña conditions (National Weather Service, 2015). Although suggestive of an effect of food availability on CORT physiology, more research on this topic is warranted.
Body condition
Chronically elevated levels of CORT are typically associated with adverse environmental influences and can have detrimental effects on individuals (Romero, 2004; Bonier et al., 2009; Blas, 2015). For example, in another closely related Neotropical–Nearctic migrant, the American redstart, elevated CORT is associated with poor-quality winter habitat in Jamaica (Marra and Holberton, 1998) that carries over to the breeding season and is linked to poor body condition (Marra et al., 1998). However, in cerulean warblers, the reverse was true; relatively high CORTf from winter was correlated with improved body condition on the breeding grounds. This pattern appears to be driven mostly by ASY males (despite a lack of significant interaction with age), which had lower CORT overall so older birds may be better able, physiologically, to capitalize on the benefits of elevated CORT (e.g. increased foraging; see Fairhurst et al., 2014). Unlike territorial American redstarts in Jamaica, cerulean warblers appear to be, at most, semi-territorial during the winter, and are often associated with mixed feeding flocks (Jones et al., 2002; Muñoz and Colorado, 2012). Thus, less competitive exclusion may occur in cerulean warblers, and habitat-driven CORT physiology may not be as crucial as it could be for more territorial species that may be relegated to a relatively small space on the wintering grounds (such as American redstarts in Jamaica).
The fine-scale timing of moult could help explain the characterization of elevated CORT as a beneficial physiological response (as may be inferred by the positive relationship between CORT and body condition, particularly for older birds) or as an indicator of poor foraging conditions/abilities (as may be inferred from the SY birds having higher CORT). Although broad patterns of moult in this species are now fairly well understood, the exact timing and variability within and among age classes is unknown. Thus, age-specific differences in the timing of the moult could further confound our ability to discern the underlying relationships (see Romero and Fairhurst, 2016 for a discussion). If pre-alternate moult occurs closer to the end of the winter season in older birds (e.g. March) when individuals are preparing physiologically for a costly migration, higher CORT concentrations under any food availability regime may reflect the pre-migratory benefits of elevated CORT (e.g., hyperphagia; Lõhmus et al., 2006). However, if moult occurs earlier in winter, regardless of age, when food limitation may be less likely, elevated CORT levels might reflect occupancy of low-quality habitats or poor food availability. Moreover, if the early moult scenario is the case, the period reflected by CORTf measurements would be even further removed from the future breeding period during which we looked for evidence of carry-over effects, making CORT–fitness relationships even less likely (see next subsection).
Fitness
Despite its relationship with breeding body condition, we found no evidence that non-breeding CORTf was related to future fitness. Although physiological responses to environmental conditions on the wintering grounds may mediate how cerulean warblers transition from non-breeding to breeding, events on the breeding grounds appear to be more influential to reproduction (e.g. Boves et al., 2013). This interpretation is congruent with recent evidence from 19 migratory bird species in the UK, where climatic variation on the breeding grounds had a much greater influence on reproductive performance than did winter climate (Ockendon et al., 2013), and with results from a study that found a lack of influence of non-breeding conditions on future fitness of winchats (Saxicola rubetra) wintering in Africa and breeding in Europe (Blackburn and Cresswell, 2016). Previous evaluations of CORTf from non-breeding birds in the context of carry-over effects have also failed to find relationships with reproductive output within a single year (e.g. in great skuas, Stercorarius skua; Bourgeon et al., 2014), but others have found relationships across several years (e.g. in common eiders, S. mollissima; Harms et al., 2015) or with specific reproductive traits related to condition and feeding (Kouwenberg et al., 2013). Had we evaluated fitness carry-over effects in females (which are very difficult to capture), we might have been more likely to find a relationship between CORT and reproduction, because costs are typically greater for females. However, the relationship between winter habitat quality and reproduction is stronger in males for American redstarts (Rushing et al., 2016), possibly because reproductive output is more variable in males.
Combining our results with those of others, the role of CORT in carry-over effects appears to be species and context specific. In cases where strong selection occurs at each stage of the life/annual cycle, carry-over effects may be weak or absent (Senner et al., 2014), so the relative influence of CORT from non-breeding birds on future reproductive performance may be minimal. Of course, we were able to consider carry-over effects only on those birds that survived the rigours of spring migration after their pre-alternate moult. Thus, we cannot rule out the possibility that environmental conditions from previous seasons still could have induced carry-over effects. For example, prior environmental conditions may have influenced the likelihood of birds surviving the spring migration north, because migration is where most mortality of migratory songbirds is thought to occur (Sillett and Holmes, 2002) and stopover locations may be more poorly protected than breeding habitat (Mehlman et al., 2005; but see Runge et al., 2015). However, this hypothesis is untestable in this species because direct long-distance tracking of cerulean warblers (or other small migrants) during migration is not feasible with current technology. If migration is the life-history stage of greatest mortality in cerulean warblers, carry-over effects from winter may be limited to influencing survival to the breeding grounds and condition upon arrival. Individuals that do survive the winter and spring migration may arrive at their breeding grounds in a variety of physiological states, but local conditions may rapidly counteract residual effects from the non-breeding season. Thus, as long as a bird can complete the journey from South America to their North American breeding grounds, carry-over effects from wintering grounds, although potentially mediated by CORT, may be relatively short lived and have little bearing on future fitness. Studies of other species that are able to be easily and rapidly captured early in the breeding season and that allow for individual arrival dates to be estimated with certainty (e.g. prothonotary warblers, Protonotaria citrea), when potential carry-over effects may still manifest, will be helpful for testing this hypothesis.
Conservation implications
Although CORTf from the winter is useful for understanding the state in which cerulean warblers transition into breeding, and can be related to individual condition during breeding, we found no support for the hypothesis that CORT physiology from the winter carries over to affect future reproduction or survival to the extent that has been documented for some species (e.g. American redstarts). Instead, individual fitness and, scaling up, population trends of cerulean warblers may be driven to a greater extent by conditions on the breeding grounds (including forest management and landscape-level fragmentation; Thompson et al., 2012; Boves et al., 2013a, b). Fitness-related seasonal interactions may still exist, particularly from the winter to migration, but these interactions will be difficult to document in the near future. Nevertheless, our study revealed age-specific variation in CORTf during winter; therefore, understanding age-specific ecology of cerulean warblers on their wintering grounds will shed light on what specific factors drive this variation and possible carry-over effects in this species. We also suggest that future studies estimate arrival date on breeding grounds and condition upon immediate arrival because, as potentially important determinants of breeding success, these factors might be relevant to CORT-mediated carry-over effects.
Acknowledgements
We thank the many hard-working field assistants who made this research successful, particularly N. E. Boves, P. C. Massey, D. W. Raybuck and A. Langevin. We also thank two anonymous reviewers for their helpful critique of previous versions of this manuscript. Finally, we are grateful to T. Marchant for the use of her endocrine labortory. Banding was conducted under US Geological Survey permit no. 22585, and feather possession was authorized by Environment Canada/Canadian Wildlife Service permit 14-SK-SC001. This study was completed under the auspices of an institutional animal care and use committee protocol from the University of Tennessee (#561).
Funding
This work was supported by the Department of Forestry, Wildlife and Fisheries at the University of Tennessee, the Department of Biological Sciences at Arkansas State University, the University of Saskatchewan, the Tennessee Wildlife Resources Agency, National Fish and Wildlife Foundation (grant numbers 2005-0064-000, 2006-0042-000, 2007-0004-000 and 2008-0009-000), the Nature Conservancy (through a US Fish and Wildlife Service Habitat Conservation Plan planning grant with the Tennessee Wildlife Resources Agency) and the Smithsonian Migratory Bird Center.
References
- Bakermans MH, Vitz AC, Rodewald AD, Rengifo CG (2009) Migratory songbird use of shade coffee in the Venezuelan Andes with implications for conservation of Cerulean Warbler. Biol Conserv 11: 2476–2483. [Google Scholar]
- Blackburn E, Cresswell W (2016) High within‐winter and annual survival rates in a declining Afro‐Palaearctic migratory bird suggest that wintering conditions do not limit populations. Ibis 158: 92–105. [Google Scholar]
- Blas J, 2015. Stress in birds In Scanes C. ed., Sturkie's Avian Physiology, Ed 6 Academic Press, San Diego. [Google Scholar]
- Bonier F, Martin PR, Moore IT, Wingfield JC (2009) Do baseline glucocorticoids predict fitness. Trends Ecol Evol 24: 634–642. [DOI] [PubMed] [Google Scholar]
- Bortolotti GR. (2010) Flaws and pitfalls in the chemical analysis of feathers: bad news–good news for avian chemoecology and toxicology. Ecol Appl 20: 1766–1774. [DOI] [PubMed] [Google Scholar]
- Bortolotti GR, Marchant TA, Blas J, German T (2008) Corticosterone in feathers is a long-term, integrated measure of avian stress physiology. Func Ecol 22: 494–500. [Google Scholar]
- Bourgeon S, Leat EHK, Magnusdóttir E, Furness RW, Strøm H, Petersen A, Gabrielsen GW, Hanssen SA, Bustnes JO (2014) Feather corticosterone levels on wintering grounds have no carry-over effects on breeding among three populations of great skuas (Stercorarius skua). PLoS One 9: e100439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boves TJ, Buehler DA (2012) Breeding biology, behavior, and ecology of Setophaga cerulea in the Cumberland Mountains, Tennessee. Southeast Nat 11: 319–330. [Google Scholar]
- Boves TJ, Buehler DA, Sheehan J, Wood PB, Rodewald AD, Larkin JL, Keyser PD, Newell FL, George GA, Evans A et al. (2013) Emulating natural disturbances for declining late-successional species: a case study of the consequences for cerulean warblers (Setophaga cerulea). PLoS One 8: e52107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boves TJ, Buehler DA, Wood PB, Rodewald AD, Larkin JL, Keyser PD, Wigley TB (2014) Multiple plumage ornaments convey information about age and within-age-class qualities of a canopy-dwelling songbird, the Cerulean Warbler. Auk 131: 20–31. [Google Scholar]
- Buehler DA, Welton MJ, Beachy TA (2006) Predicting cerulean warbler habitat use in the Cumberland Mountains of Tennessee. J Wildlife Manag 70: 1763–1769. [Google Scholar]
- Buehler DA, Hamel PB, Boves T (2013) Cerulean warbler (Setophaga cerulea). In Poole A., ed., The Birds of North America Online. Cornell Lab of Ornithology, Ithaca; Retrieved from the Birds of North America Online, http://bna.birds.cornell.edu/bna/species/511. [Google Scholar]
- Daunt F, Wanless S, Harris MP, Money L, Monaghan P (2007) Older and wiser: improvements in breeding success are linked to better foraging performance in European shags. Func Ecol 21: 561–567. [Google Scholar]
- DeLuca WV, Woodworth BK, Rimmer CC, Marra PP, Taylor PD, McFarland KP, Mackenzie SA, Norris DR (2015). Transoceanic migration by a 12 g songbird. Biol Lett 11: 20141045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairhurst GD, Dawson RD, van Oort H, Bortolotti GR (2014) Synchronizing feather-based measures of corticosterone and carotenoid-dependent signals: what relationships do we expect. Oecol 174: 689–698. [DOI] [PubMed] [Google Scholar]
- Fairhurst GD, Bond AL, Hobson KA, Ronconi RA (2015. a) Feather-based measures reveal a relationship between trophic position and physiology in a pelagic seabird over a 153-year period. Ibis 157: 273–283. [Google Scholar]
- Fairhurst GD, Berzins LL, Bradley DW, Laughlin AJ, Romano A, Romano M, Scandolara C, Ambrosini R, Dawson RD, Dunn PO et al. (2015. b) Assessing costs of carrying geolocators using feather corticosterone in two species of aerial insectivore. Roy Soc Open Sci 2: 150004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garreaud RD. (2009) The Andes climate and weather. Adv Geosci 22: 3–11. [Google Scholar]
- Grunst ML, Grunst AS, Parker CE, Romero LM, Rotenberry JT (2015) Pigment-specific relationships between feather corticosterone concentrations and sexual coloration. Behav Ecol 26: 706–715. [Google Scholar]
- Harrison XA, Blount JD, Inger R, Norris DR, Bearhop S (2011) Carry-over effects as drivers of fitness differences in animals. J Anim Ecol 80: 4–18. [DOI] [PubMed] [Google Scholar]
- Harms NJ, Legagneux P, Gilchrist HG, Bêty J, Love OP, Forbes MR, Bortolotti GR, Soos C (2015) Feather corticosterone reveals effect of moulting conditions in the autumn on subsequent reproductive output and survival in an Arctic migratory bird. Proc Biol Sci 282: 20142085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins GL, Hill GE, Mercadante A (2012) Delayed plumage maturation and delayed reproductive investment in birds. Biol Rev 87: 257–274. [DOI] [PubMed] [Google Scholar]
- Jones J, Ramoni-Perazzi P, Carruthers EH, Robertson RJ (2002) Species composition of bird communities in shade coffee plantations in the Venezuelan Andes. Ornitologia Neotropical 13: 397–412. [Google Scholar]
- Kitaysky AS, Piatt JF, Hatch SA, Kitaiskaia EV, Benowitz-Fredericks ZM, Shultz MT, Wingfield JC (2010) Food availability and population processes: severity of nutritional stress during reproduction predicts survival of long-lived seabirds. Funct Ecol 24: 625–637. [Google Scholar]
- Klaassen RHG, Hake M, Standberg R, Koks BJ, Trierweiler C, Exo K-M, Bairlein F, Alerstam T (2014) When and where does mortality occur in migratory birds? Direct evidence from long-term satellite tracking of raptors. J Anim Ecol 83: 176–184. [DOI] [PubMed] [Google Scholar]
- Kouwenberg A-L, Hipfner JM, McKay DW, Storey AE (2013) Corticosterone and stable isotopes in feathers predict egg size in Atlantic Puffins Fratercula arctica. Ibis 155: 413–418. [Google Scholar]
- Krist M. (2011) Egg size and offspring quality: a meta-analysis in birds. Biol Rev 86: 692–716. [DOI] [PubMed] [Google Scholar]
- Lendvai AZ, Giraudeau M, Nemeth J, Bako V, McGraw KJ (2013) Carotenoid-based plumage coloration reflects feather corticosterone levels in male house finches (Haemorhous mexicanus). Behav Ecol Sociobiol 67: 1817–1824. [Google Scholar]
- Lendvai AZ, Giraudeau M, Bokony V, Angelier F, Chastel O (2015) Within-individual plasticity explains age-related decrease in stress response in a short-lived bird. Biol Lett 11: 20150272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lõhmus M, Sundström LF, Moore FR (2006) Non-invasive corticosterone treatment changes foraging intensity in red-eyed vireos Vireo olivaceus. J Avian Biol 37: 523–526. [Google Scholar]
- McKim-Louder MI, Hoover JP, Benson TJ, Schelsky WM (2013) Juvenile survival in a Neotropical migratory songbird is lower than expected. PLoS One 8: e56059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marra PP, Holberton RL (1998) Corticosterone levels as indicators of habitat quality: effects of habitat segregation in a migratory bird during the non-breeding season. Oecologia 116: 282–294. [DOI] [PubMed] [Google Scholar]
- Marra PP, Hobson KA, Holmes RT (1998) Linking winter and summer events in a migratory bird by using stable-carbon isotopes. Science 282: 1884–1886. [DOI] [PubMed] [Google Scholar]
- Marra PP, Cohen EB, Loss SR, Rutter JE, Tonra CM (2015) A call for full annual cycle research in animal ecology. Biol Lett 11: 20150552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin K. (1995) Patterns and mechanisms for age-dependent reproduction and survival in birds. Am Zool 35: 340–348. [Google Scholar]
- Mehlman DW, Mabey SE, Ewert DN, Duncan C, Abel B, Cimprich D, Sutter RD, Woodrey M (2005) Conserving stopover sites for forest-dwelling migratory landbirds. Auk 122: 1281–1290. [Google Scholar]
- Muñoz JM, Colorado GJ (2012). Foraging ecology of the Cerulean Warbler (Setophaga cerulea) in Andean agroforestry ecosystems. Ornitol Neotrop 23: 359–366. [Google Scholar]
- National Weather Service (2015) Climate Prediction Service: Historical El Niño/La Niña episodes (1950–present). www.cpc.ncep.noaa.gov/products/analysis_monitoring/ensostuff/ensoyears.shtml.
- Norris DR, Marra PP (2007) Seasonal interactions, habitat quality, and population dynamics in migratory birds. Condor 109: 535–547. [Google Scholar]
- Norris DR, Marra PP, Kyser TK, Sherry TW, Ratcliffe LM (2004) Tropical winter habitat limits reproductive success on the temperate breeding grounds in a migratory bird. Proc Biol Sci 271: 59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ockendon N, Leech D, Pearce-Higgins JW (2013) Climatic effects on breeding grounds are more important drivers of breeding phenology in migrant birds than carry-over effects from wintering grounds. Biol Lett 9: 20130669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang JQ, Hau M, Bonier F (2011) Within seasons and among years: when are corticosterone levels repeatable. Horm Behav 60: 559–564. [DOI] [PubMed] [Google Scholar]
- Pyle P. (1997) Identification Guide to North American Birds. Slate Creek Press, Bolinas, CA, USA. [Google Scholar]
- Romero LM. (2004) Physiological stress in ecology: lessons from biomedical research. Trends Ecol Evol 19: 249–255. [DOI] [PubMed] [Google Scholar]
- Romero LM, Fairhurst GD (2016). Measuring corticosterone in feathers: strengths, limitations, and suggestions for the future . Comp Biochem Physiol A Mol Integr Physiol In press. 10.1016/j.cbpa.2016.05.002. [DOI] [PubMed] [Google Scholar]
- Runge CA, Watson WEM, Butchart SHM, Hanson JO, Possingham HP, Fuller RA (2015) Protected areas and global conservation of migratory birds. Science 350: 1255–1258. [DOI] [PubMed] [Google Scholar]
- Rushing CS, Marra PP, Dudash MR (2016) Winter habitat quality but not long-distance dispersal influences apparent reproductive success in a migratory bird. Ecology 5: 1218–1227. [DOI] [PubMed] [Google Scholar]
- Salton M, Saraux C, Dann P, Chiaradia A (2015) Carry-over body mass effect from winter to breeding in a resident seabird, the little penguin. Roy Soc Open Sci 2: 140390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer JR, Hines JE, Fallon JE, Pardieck KL, Ziolkowski DJ Jr, Link WA (2014) The North American Breeding Bird Survey, Results and Analysis 1966–2013. Version 01.30.2015. USGS Patuxent Wildlife Research Center, Laurel, MD, USA. [Google Scholar]
- Schamber JL, Esler D, Flint PL (2009) Evaluating the validity of using unverified indices of body condition. J Avian Biol 40: 49–56. [Google Scholar]
- Schulte-Hostedde AI, Zinner B, Millar JS, Hickling GJ (2005) Restitution of mass-size residuals: validating body condition indices. Ecology 86: 155–163 [Google Scholar]
- Senner NR, Hochachka WM, Fox JW, Afanasyev V (2014). An exception to the rule: carry-over effects do not accumulate in a long-distance migratory bird. PLoS One 9: e86588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillett TS, Holmes RT (2002) Variation in survivorship of a migratory songbird throughout its annual cycle. J Anim Ecol 71: 296–308. [Google Scholar]
- Sorensen MC, Hipfner JM, Kyser TK, Norris DR (2009) Carry‐over effects in a Pacific seabird: stable isotope evidence that pre‐breeding diet quality influences reproductive success. J Anim Ecol 78: 460–467. [DOI] [PubMed] [Google Scholar]
- Studds CE, Marra PP (2005) Nonbreeding habitat occupancy and population processes: an upgrade experiment with a migratory songbird. Ecology 86: 2380–2385. [Google Scholar]
- Stutchbury BJM, Tarof SA, Done T, Gow E, Kramer PM, Tautin J, Fox JW, Afanasyev V (2009) Tracking long-distance songbird migration by using geolocators. Science 323: 896–896. [DOI] [PubMed] [Google Scholar]
- Thompson FR III, Robbins MB, Fitzgerald JA (2012) Landscape-level forest cover is a predictor of Cerulean Warbler abundance. Wilson J Ornithol 124: 721–727. [Google Scholar]
- Tonra CM, Marra PP, Holberton RL (2014) Experimental and observational studies of seasonal interactions between overlapping life history stages in a migratory bird. Horm Behav 64: 825–832. [DOI] [PubMed] [Google Scholar]
- Van Bael SA, Aiello A, Valderrama A, Medianero E, Samaniego M, Wright SJ (2004) General herbivore outbreak following an El Niño-related drought in a lowland Panamanian forest. J Trop Ecol 20: 625–633. [Google Scholar]
- Wayland M, Gilchrist HG, Marchant T, Keating J, Smits JE (2002) Immune function, stress response, and body condition in Arctic-breeding common eiders in relation to cadmium, mercury, and selenium concentrations. Environ Res 90: 47–60. [DOI] [PubMed] [Google Scholar]
- Webster MS, Marra PP (2005) The importance of understanding migratory connectivity and seasonal interactions In Greenberg R, Marra PP, eds, Birds of Two Worlds: the Ecology and Evolution of Migration. Johns Hopkins University Press, Baltimore, pp 199–209. [Google Scholar]
- Wingfield JC, Kitaysky AS (2002) Endocrine responses to unpredictable environmental events: stress or anti-stress hormones. Integr Comp Biol 42: 600–609. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Sapolsky R (2003) Reproduction and resistance to stress: when and how. J Neuroendocrinol 15: 711–724. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Moore MC, Farner DS (1983) Endocrine responses to inclement weather in naturally breeding populations of white-crowned sparrows (Zonotrichia leucophrys pugetensis). Auk 100: 56–62. [Google Scholar]
- Wunderle JM. (1991) Age-specific foraging proficiency in birds. Curr Ornithol 8: 273–324. [Google Scholar]