Fig 1. Identification of a GD2-specific binding peptide and binding characteristics of WHWRLPS-phage in vitro.
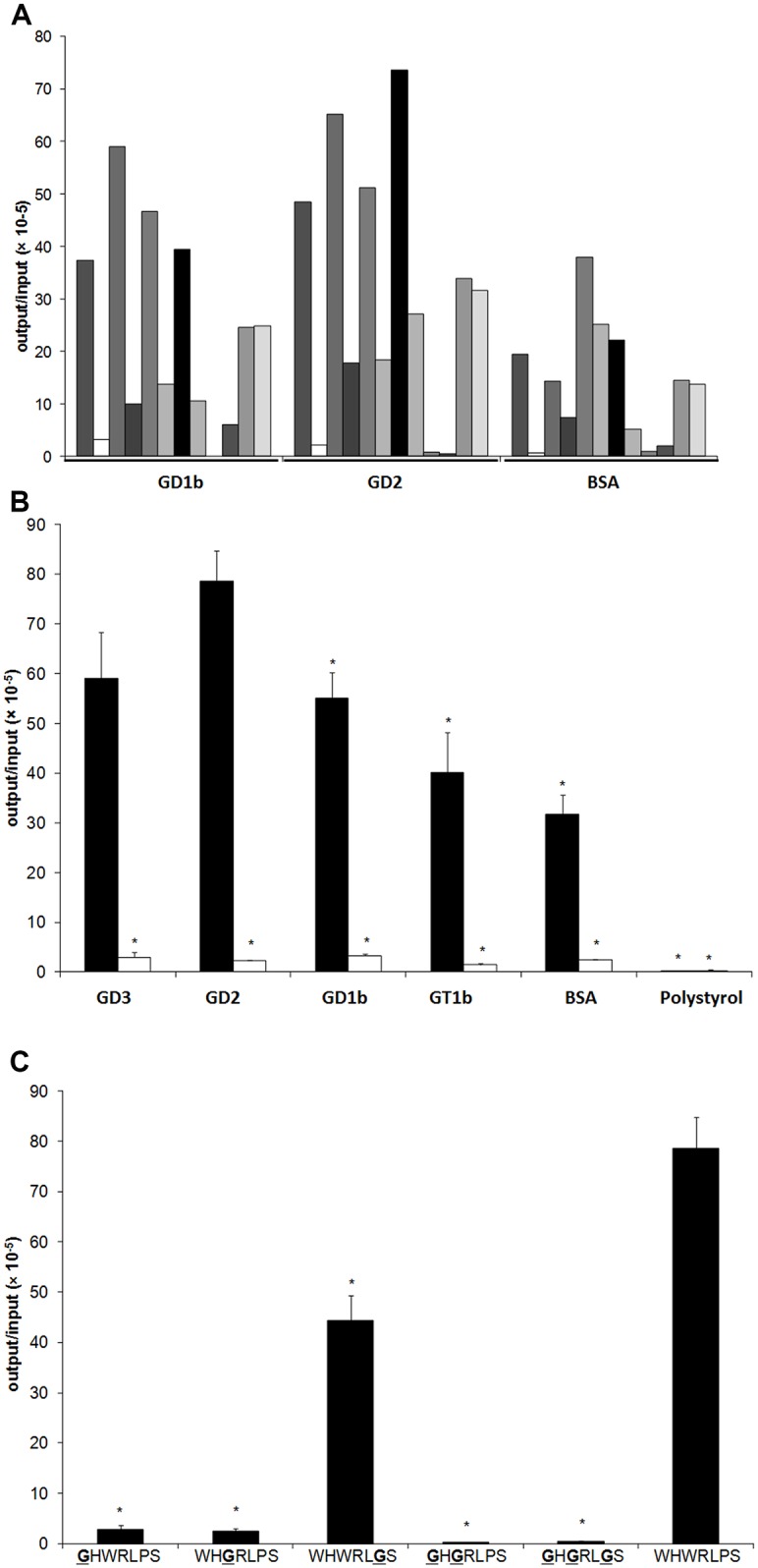
A) Individual phage clones (n = 12) derived from a phage display screen were tested for binding to immobilized human GD1b, immobilized human GD2, or to BSA-coated surfaces. Phage clone 7 displayed the peptide sequence WHWRLPS and bound with high affinity to human GD2, and to a lesser extent, to human GD1b. B) Binding affinity of WHWRLPS-phage (black bars) to GD3, GD2, GD1b, GT1, BSA or polystyrol was tested and compared to control phage (white bars). “*” = p < 0.05 vs. GD2 binding of the WHWRLPS-phage. C) Mutations in the displayed heptapeptide and resulting affinities to GD2. * p < 0.01 vs. unmutated WHWRLPS-phage.
