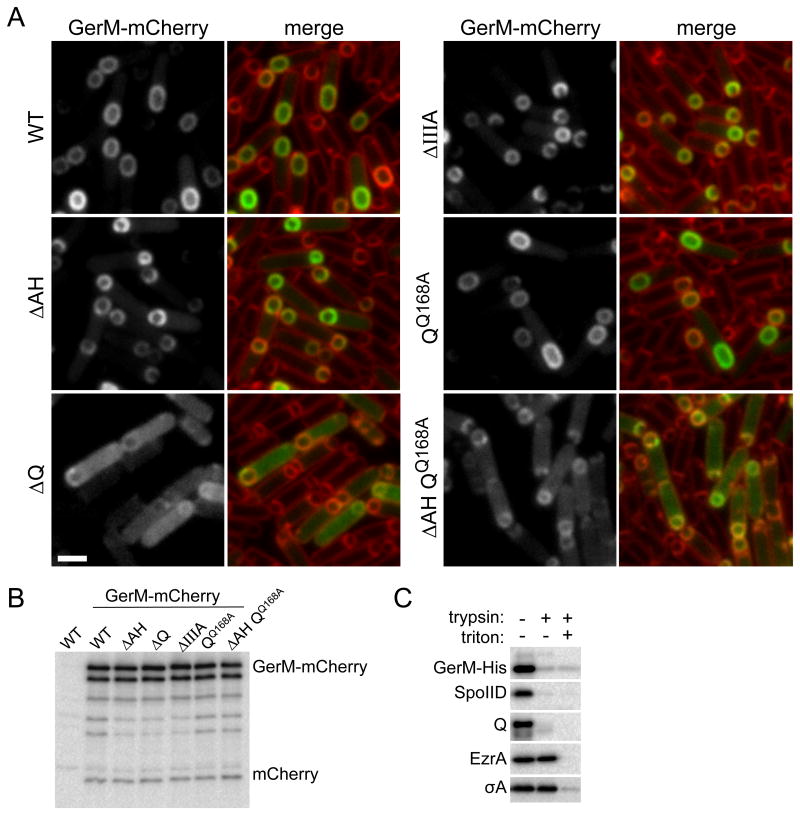
Figure 4. GerM-mCherry localizes to septal membrane in a manner that depends on Q.
A. Representative images of GerM-mCherry localization at 2.5 hours after the onset of sporulation. Images are from wild-type (BCR1332), ΔAH (BCR1344), ΔQ (BCR1345), ΔspoIIIA (BCR1346), QQ168A (BCR1348) and the ΔAH QQ168A double mutant (BCR1353). Scale bar represents 2 μm. B. GerM-mCherry levels and proteolytic products are similar in all mutant backgrounds tested. Immunoblot analysis using anti-mCherry antibodies of sporulating cells from the same strains described above and with a true wild-type control (PY79, no mCherry) harvested at hour 2.5 of sporulation. The nature of the GerM-mCherry doublet is currently unknown. C. GerM-His6 is surface exposed and thus accessible to trypsin digestion. Immunoblot analysis using anti-His antibodies of protoplasted sporulating cells (strain BCR1306) treated with Trypsin in the presence and absence of TritonX-100. Consistent with the idea that GerM is a lipoprotein, it remained cell-associated after the generation of protoplasts. As controls, the immunoblot was probed for two membrane proteins with extracellular domains (SpoIID and Q), a membrane-anchored cytoplasmic protein (EzrA), and a cytoplasmic protein (SigA).