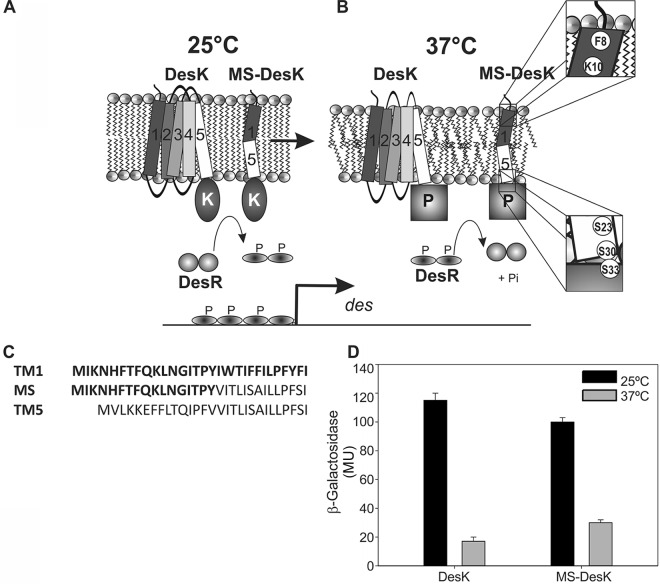
FIG 1.
Cold detection in B. subtilis. (A) The histidine kinase DesK (five TM-spanning segments) and the synthetic MS-DesK protein localize at the lipid bilayer. At low temperature (25°C), the level of order in the structures of the acyl chains of lipids increases, promoting a kinase-active state. DesK autophosphorylates and transfers the phosphoryl group to Asp54 of the DesR dimeric response regulator (two gray circles), inducing a conformational change that results in exposure of the helix-turn-helix (HTH)–DNA-binding domain. Two dimers of phosphorylated DesR bind two adjacent, nonidentical binding sites within the des promoter (40), leading to recruitment of the RNA polymerase to activate expression of the desaturase gene. (B) At higher temperature (37°C), the disordered lipids favor the phosphatase-active state of DesK, leading to dephosphorylation of phospho-DesR; thus, des transcription is turned off. The dyad Phe8-Lys10 and the serine zipper are highlighted in the insets to show their locations at the water-lipid interphase. (C) Sequence of the functional synthetic TMS of minimal sensor DesK and the nonfunctional TM1 and TM5. (D) DesK and MS kinase activities at 25°C and 37°C. MU, Miller units. (Adapted from reference 10 with permission of the publisher.)