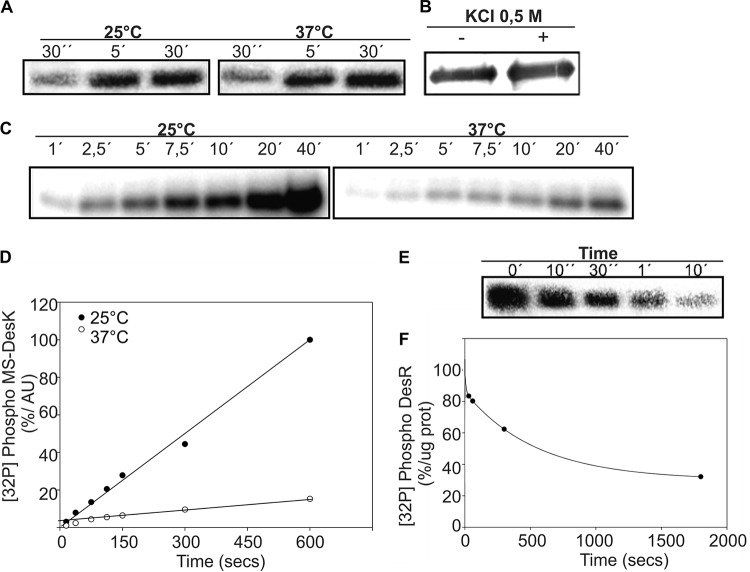
FIG 3.
MS-DesK reconstituted in liposomes displays autokinase and phosphatase activities. (A) The autokinase activity of detergent-dissolved MS-DesK is not regulated by temperature. MS-DesK solubilized in 0.5% Brij 58 was incubated with [γ-32P]ATP at either 25 or 37°C, and the autokinase activity was analyzed at different interval points by SDS-PAGE followed by autoradiography. (B) DesK is integrated into liposomes. Proteoliposomes were purified by a sucrose gradient centrifugation in the absence (–) or presence (+) of 0.5 M KCl and later analyzed by Western blotting using anti-His antibodies. (C) MS-DesK integrated into liposomes was incubated with [γ-32P]ATP at either 25 or 37°C, and the autokinase activity was measured by taking samples at different time points. The autokinase activity of proteoliposomes is regulated by temperature, showing that MS-DesK must be integrated into a lipid bilayer to be thermoregulated. (D) Quantification of the MS-DesK kinase activity shown in panel C by the use of Image Quant software (version 5.2). AU, arbitrary units. (E) MS-DesK phosphatase activity. Proteoliposomes containing MS-DesKC were incubated at 37°C with purified DesR-P. The dephosphorylation reactions were analyzed by SDS-PAGE followed by autoradiography. (F) The total amount of DesR-P (expressed in arbitrary units) present in each well was determined by densitometry as described for panel D; the total labeling of DesR-P at the beginning of the reaction (0 min) was considered 100%. The graph shows the percentage of DesR-P protein (prot) remaining as a function of time.