FIG 5.
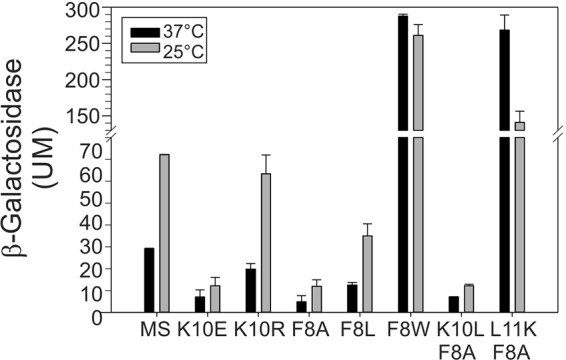
The dyad F8-K10 is critical for determining the signaling state of MS-DesK. Different MS-DesK variants with mutations in the dyad F8-K10 were analyzed for in vivo activity. The conservative replacement K10R does not eliminate thermoregulation of MS-DesK activity; however, the replacement of K10L and K10E abolished high kinase activity at low temperatures, suggesting that a positive charge is required at position 10. The conservative replacement F8L also maintains regulation, as expected, because leucine and phenylalanine have the same hydrophobic profile. F8A behaves much like K10E, probably because of the loss of hydrophobicity at residue 8. F8W is constitutively active due to the strong preference of Trp for the water-lipid interphase. The double mutant F8A-K10L lacks kinase activity, since both components of the thermosensitive dyad are eliminated. The double mutant F8A-L11K is also constitutively active; since the presence of an extra lysine residue located deeper (1.5 Å) inside the membrane exerts an outward pull on the TMS even at higher temperatures, when the membrane is thinner. β-Galactosidase activity was determined as described for Fig. 4B.
