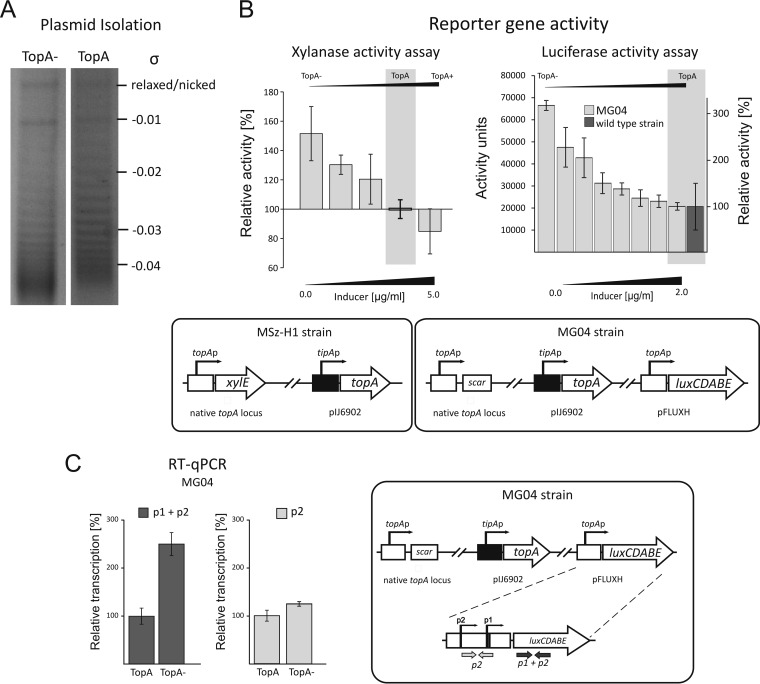
FIG 3.
Induction of the topA promoter by high chromosomal supercoiling density. (A) Analysis of the supercoiling density (σ) of the reporter plasmid pWHM3Hyg isolated from strains with a wild-type (TopA) or depleted (TopA−) level of topoisomerase I. (B) (Left) Relative xylanase activities in MSz-H1 strain cell extracts (see the scheme at bottom) cultured under different inducing conditions, i.e., high, wild-type, and low DNA supercoiling densities. Different supercoiling conditions were achieved via the induction of tipAp-controlled topA expression with 0 to 5 μg/ml of thiostrepton, as indicated. The wild-type level of TopA protein corresponds to an inducer concentration of 2 μg/ml, whereas lower and higher inducer concentrations led to TopA depletion (TopA−) and overproduction (TopA+), respectively. (Right) Relative luciferase activities in the MG04 strain (see the scheme at bottom) cultured under conditions inducing high supercoiling density. High supercoiling in MG04 was achieved by the induction of tipAp-controlled topA gene expression with thiostrepton at concentrations of up to 2 μg/ml (wild-type level of TopA protein) and was compared to that of the wild-type strain (MG03). (C) Levels of topA promoter-driven transcripts in the MG04 strain (see scheme at right) with TopA depletion (TopA−) and wild-type TopA levels (TopA). The overall topA promoter activity (p1 + p2) was quantified by detection of the luxC transcript (left), whereas p2-specific transcription (p2) was quantified using oligonucleotides specific to the topA promoter region (right).