Fig. 5.
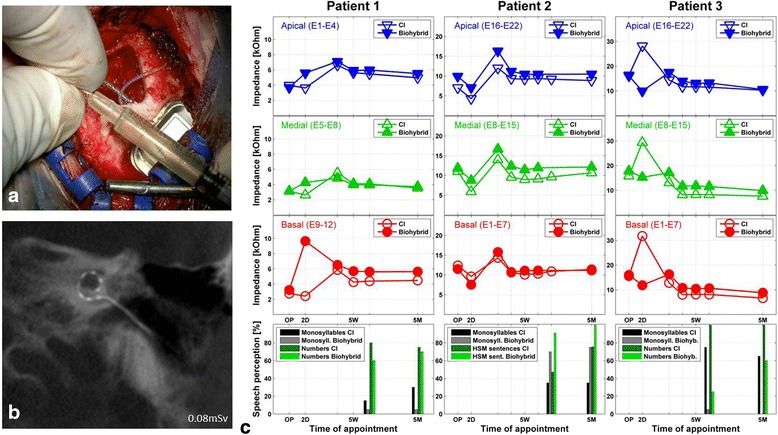
Generation of a biohybrid electrode for human implantation and comparison of performance with standard (CI) and cell-coated electrodes (biohybrid). a Dipping procedure for the intraoperative coating of the electrode with a fibrin cell layer prior to implantation. The BM-MNC solution was mixed with the fibrinogen solution and used for the dip-coating of the electrode. A second dipping into the thrombin solution allowed the stabilization of the coating by conversion of the fibrinogen to fibrin. b Cone-beam computed tomography was utilized to check the electrode position immediately after insertion in the operating room. The micrograph shows the correct intracochlear position of the electrode array without any displacement. c (Left panels) Impedances and speech perception (monosyllables and numbers) of patient one. The patient received a MedEl Synchrony Standard electrode and showed an overall good performance; slightly impaired results on the side with long-term deafness that was treated with the biohybrid electrode are evident. (Middle panels) Impedances and speech perception (monosyllables and HSM sentence test) of patient two. The patient received a Cochlear Nucleus CI512 Profile. The results compared favourably to the contralateral side. (Right panels) Impedances and speech perception (monosyllables and numbers) of patient three. The patient received a Cochlear Nucleus CI512 Profile electrode. His results with the biohybrid electrode exceeded expectations, taking into consideration the presence of peri/prelingual idiopathic deafness on the side that was treated with the biohybrid electrode. OP operation, 2D initial test (only impedances) on the second day after surgery, 5 W first fitting week performed 5 weeks after operation, 5 M control testing 5 months after operation, respectively about three months after the first fitting
