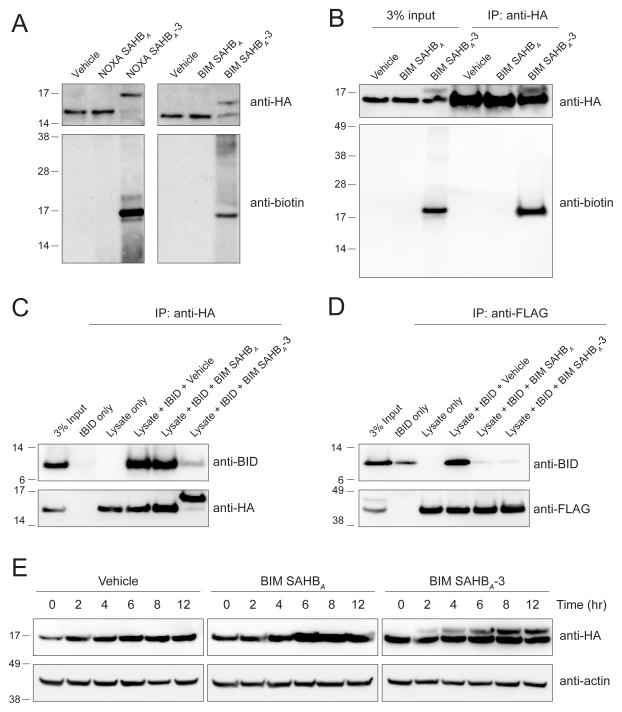
Figure 4. Covalent Targeting of BFL-1 C55 in Lysates and Cells.
(A) Biotinylated NOXA and BIM SAHBA-3 peptides crosslinked to HA-BFL-1ΔC C4S/C19S in lysates from transfected 293T lysates, as evidenced by the shift in molecular weight of BFL-1ΔC observed upon anti-HA western analysis. Anti-biotin blotting confirmed the selective incorporation of biotin into the HA-BFL-1ΔC band, with little to no crossreactivity with other proteins in the cellular lysate.
(B) Treatment of transfected 293T cells with biotinylated BIM SAHBA-3 followed by cellular lysis, HA immunoprecipitation, and biotin western analysis demonstrated the capacity of a warhead-bearing BIM SAHB to gain intracellular access and covalently target expressed HA-BFL-1ΔC C4S/C19S containing the native C55.
(C–D) BIM SAHBA-3, but not BIM SAHBA, effectively competed with tBID for HA-BFL-1ΔC C4S/C19S interaction in 293T lysates, achieving robust covalent conjugation (C), whereas in the context of exclusive non-covalent FLAG-MCL-1 interaction, the compounds were equally effective at competing with tBID (D), as measured by the indicated immunoprecipitation and western analyses.
(E) Covalent modification of HA-BFL-1ΔC C4S/C19S by cellular treatment with BIM SAHBA-3, but not the corresponding construct lacking the acrylamide-based warhead. Crosslinked BFL-1ΔC was observed by 2 hours and levels continued to increase in a time-dependent fashion throughout the 12 hour treatment period, as monitored by HA western analysis.
See also Figure S2.