Abstract
Background and Aims Traits related to flower advertisement and reward sometimes vary in a circadian way, reflecting phenotypic specialization. However, specialized flowers are not necessarily restricted to specialized pollinators. This is the case of most Silene species, typically associated with diurnal or nocturnal syndromes of pollination but usually showing complex suites of pollinators.
Methods A Silene species with mixed floral features between diurnal and nocturnal syndromes was used to test how petal opening, nectar production, scent emission and pollination success correlate in a circadian rhythm, and whether this is influenced by environmental conditions. The effect of diurnal and nocturnal visitation rates on plant reproductive success is also explored in three populations, including the effect of the pollinating seed predator Hadena sancta.
Key Results The result showed that repeated petal opening at dusk was correlated with nectar secretion and higher scent production during the night. However, depending on environmental conditions, petals remain opened for a while in the morning, when nectar and pollen still were available. Pollen deposition was similarly effective at night and in the morning, but less effective in the afternoon. These results were consistent with field studies.
Conclusions The circadian rhythm regulating floral attractiveness and reward in S. colorata is predominantly adapted to nocturnal flower visitors. However, favourable environmental conditions lengthen the optimal daily period of flower attraction and pollination towards morning. This allows the complementarity of day and night pollination. Diurnal pollination may help to compensate the plant reproductive success when nocturnal pollinators are scarce and when the net outcome of H. sancta shifts from mutualism to parasitism. These results suggest a functional mechanism explaining why the supposed nocturnal syndrome of many Silene species does not successfully predict their pollinator guilds.
Keywords: Flower scent, Hadena, nectar production, nursery pollination, nyctinasty, pollination syndrome
INTRODUCTION
Plant biological rhythms influence the physiology of individuals and have evolved to enhance fitness in response to environmental changes (McClung, 2006; Sanchez et al., 2011). The circadian rhythms of flowers (mediated by light–dark cycles) include flower opening and closure movements (Linné’s floral clock: Linneaus, 1783; van Doorn and van Meeteren, 2003; McClung, 2006), timing of nectar production (Cruden et al., 1983) and diel variation of flower scent (Dobson, 2006; Knudsen et al., 2006). Floral nyctinasty, one of the rhythmic movements of plant organs in response to the onset of darkness (Darwin and Darwin, 1880; Palmer and Asprey, 1958; Satter and Galston, 1981), is the repeated opening of flowers in the evening/night. It has long been presumed that flower nyctinasty as well as dynamics of nectar secretion and scent emission have evolved to match the time of activity of the most important pollinators (Dudareva et al., 2000a; van Doorn and van Meeteren, 2003; Pacini and Nepi, 2007). These flower traits are important components of pollination syndromes, defined as the suite of floral traits that have independently evolved in different plant lineages due to the convergent selection by specific groups of pollinators (Faegri and van der Pijl, 1979; Fenster et al., 2004; Vogel, 2006; Ollerton et al., 2009).
The typological nature of the pollination syndrome concept has been controversial since its formulation (see Waser et al., 2011). More recently, the criticisms of syndromes arose because phenotypic specialized flowers are not necessarily restricted to specialized pollinators (Waser et al., 1996; Ollerton et al., 2007; Armbruster, 2014), although recent works suggest that pollination syndromes accurately predict the most effective pollinators (Reynolds et al., 2009; Rosas-Guerrero et al., 2014; but see Ollerton et al., 2015 and subsequent responses). It has been suggested that the dilemma of specialized flowers with generalized pollination may be partially explained by the lack of fitness trade-offs (Aigner, 2001, 2004). Phenotypic specialization of flowers may incur fitness trade-offs when the positive effect of specialized flower traits on effectiveness of one group of pollinators is linked to reduced effectiveness of other pollinators (Galen and Newport, 1987; Hurlbert et al., 1996; Miller et al., 2014), or higher susceptibility to specialized herbivores and pathogens (Strauss and Whittall, 2006). When fitness trade-offs are absent or weak, phenotypic specialization of flowers can be maintained without reducing the functional diversity of pollinators (Armbruster, 2014). These reasons, together with the spatio-temporal variation in the abundance and identity of the most effective pollinators, may explain the prevalence of generalized over specialized pollination systems from an evolutionary perspective (Herrera, 1996; Waser et al., 1996; Johnson and Steiner, 2000; Fenster et al., 2004; Gómez and Zamora, 2006; Waser and Ollerton, 2006).
The genus Silene L. (Caryophyllaceae) is a model system for studies in ecology and evolution (Bernasconi et al., 2009) and is characterized by its diversity of floral phenotypes. In Silene, two contrasting flower phenotypes have been traditionally described, namely nocturnal and diurnal (Lindman, 1897; Greuter, 1995). ‘Diurnal’ species usually have pink or red petals, and flowers are usually open during the day and night. These species do not show obvious changes of scent intensity between day and night as perceived by the human nose (Greuter, 1995; Jürgens, 2004, 2006). These correlated flower traits are indicative of both long-tongued bees and diurnal Lepidoptera syndromes (Fenster et al., 2004; Reynolds et al., 2009). ‘Nocturnal’ species have white or pale flowers that show repeated petal opening and intense scent emission in the evening/night (Greuter, 1995; Jürgens et al., 2002; Jürgens, 2006; Castillo et al., 2014; Buide et al., 2015), so they are suggestive of nocturnal moth syndrome (Faegri and van der Pijl, 1979; Jürgens et al., 2002; Giménez-Benavides et al., 2007; Reynolds et al., 2009; Martinell et al., 2010). However, many studies suggest that almost every Silene species is visited by diurnal and nocturnal insects (Jürgens, 2004; Jürgens et al., 2002; Kephart et al., 2006; Reynolds et al., 2009; Buide et al., 2015).
Flower nyctinasty has been barely studied in Silene and, although there are many studies dealing with the flower specialization of Silene species, some questions remain unclear. First, few case studies have addressed whether presumed pollination syndromes of Silene species accurately predict the most effective pollinators (Giménez-Benavides et al., 2007; Reynolds et al., 2009; Martinell et al., 2010). Secondly, some flower traits have received less attention despite the fact that they may be also regulated by circadian rhythms, such as the dynamics of nectar secretion, anther dehiscence, pollen viability and stigmatic receptivity (Bassani et al., 1994; Witt et al., 1999; Buide and Guitán, 2002; Young and Gracvits, 2002). Thirdly, it is not clear whether the daily variation in these advertisement and reward traits affects the interaction between Silene species and their specialist nursery pollinators. The moths of the genus Hadena (Noctuidae) pollinate many Silene species, but also use the flowers and developing fruits as a food resource for their larval offspring. The outcome of this interaction may shift between mutualistic and antagonistic depending on the presence and importance of other pollinators (Giménez-Benavides et al., 2007; Reynolds et al., 2012). For these reasons, the Silene–Hadena system has emerged as a good model system to understand the evolution of mutualisms (Kephart et al., 2006).
In this study, we evaluate the functional coherence of flower traits exhibited by S. colorata, an interesting species with combined floral features of both diurnal and nocturnal syndromes. Silene colorata has flowers with pink petals, but shows a marked nyctinasty and emits flower scent during the night but not at mid-day (Prieto-Benítez et al., 2015). The closure of petals does not prevent visits by diurnal pollinators, as shown in other Silene species (Giménez-Benavides et al., 2007; Martinell et al., 2010). Our specific objectives were: (1) to characterize the diel variation of traits related to flower attractiveness to pollinators (petal opening, emission of scent and secretion of nectar); (2) to analyse its breeding system and to assess whether anther dehiscence and pollination success are synchronized with flower nyctinasty; (3) to determine whether floral specialization in S. colorata may incur fitness trade-offs between day and night pollination; and (4) to explore the effect of diurnal and nocturnal flower visitors on plant reproductive success in natural populations, including the interaction with its Hadena nursery pollinator.
MATERIALS AND METHODS
Plant material
Silene colorata Poiret (Caryophyllaceae) is an annual plant with a height of 15–60 cm. The calyx is 10–15 mm in length and petal limbs are 5–12 mm, bipartite and pink. Fruit capsules open at the top when ripe and hold 45–85 seeds of 1–1·5 mm in diameter (Talavera, 1990). Flowers are protandrous, and anthesis (first opening of the flower from the bud stage) is at sunset. The petal limbs remain open all night and close (rolling themselves up) early in the morning. Nonetheless, the sexual parts of flowers remain accessible when petals are completely rolled up (pers. obs.). This species inhabits croplands and roadsides of the Mediterranean region, north of Iran, Arabia and the Canary Islands (Talavera, 1990). In our area of study (Madrid, Spain) the flowering period usually spans from April to June.
Plants used in this study grew from seeds in the greenhouse of the Universidad Rey Juan Carlos (Móstoles, Madrid 40º20′02″N, 3º52′57″W, altitude 651 m). Seeds were obtained directly from natural populations in summer 2011 and 2012 (Supplementary Data Table S1) and stored in silica gel at ambient temperature until the following spring, when they were sown in 5 cm seedling trays. After 3 months, plantlets were transferred to 2 L pots until flowering. Plants grew outdoors in an insect exclusion cage from June to July in 2012 and 2013. Pollinator observations were done in the populations of origin.
Effect of light intensity and soil moisture on timing and duration of flower opening
Since petal nyctinasty is related to water content in limb cells (Halket, 1931), we expected that plants exposed to high light intensity and/or dry soil close their petals earlier in the morning, and open them later in the evening, compared with those exposed to low light intensity and/or wetter soil. To explore this, we subjected potted plants to a factorial experiment with two levels of light intensity and two levels of soil moisture. The initial number of plants was equal for all treatments but a failure in the irrigation system left the experiment as follows: ‘Shade–Wet’ (n = 8), ‘Shade–Dry’ (n = 4), ‘Sun–Wet’ (n = 8) and ‘Sun–Dry’ (n = 8). ‘Wet’ plants were supplied with 60 min of drip irrigation every day, and ‘Dry’ plants every 2 d. ‘Sun’ plants were exposed to direct solar radiation, whereas ‘Shade’ plants were placed under a shading net. The light intensity was 191·25 and 42·25 μmol photon/m2/s in the ‘Sun’ and ‘Shade’ treatments, respectively (mean of 2 d at 0800, 1630 and 2030 h, with a Field Scout Quantum Light Meter; Spectrum Technologies, Plainfield, USA). In the ‘Sun’ treatment, the temperature varied between 21·8 and 39·3 ºC in the morning (0730 to 1100 h) and between 31·8 and 23·1 ºC during the evening/night (2030–0000 h). In the ‘Shade’ treatment, the temperature varied between 21·9 and 32·6 ºC in the morning and between 31·9 and 24 ºC during the evening/night.
The dynamics of petal opening and closure at dusk and dawn were calculated by measuring the corolla diameter every 30 min, from 2030 to 0000 h and from 0730 to 1100 h, respectively. We measured 154 flowers (in total, 1731 measurements) with a digital caliper from 11 to 18 July 2013. The mean ± s.e. of flowers per plant used in each treatment were: ‘Shade–Wet’ 9·0 ± 2·7, ‘Shade–Dry’ 6·8 ± 2·8, ‘Sun–Wet’ 9·2 ± 1·0 and ‘Sun–Dry’ 9·6 ± 2·6. In each flower, the maximum diameter achieved was considered as 100 % of the corolla opening, and was used to calculate the percentage flower opening during each time interval.
Dynamics of flower scent emission
Previous analysis reported that S. colorata did not emit scent at mid-day, unlike the typical Silene species with diurnal pollination syndrome (Prieto-Benítez et al., 2015). However, we wanted to assess whether S. colorata emits flower scent at the beginning of the day and, in that case, to compare the emission rate and composition with nocturnal samples. From 11 to 18 July 2013, we sampled flower volatile organic compounds (VOCs) from overall 11 plants using a dynamic head-space method. Inflorescences were enclosed in polyethylene oven bags for 5 min, and the emitted volatiles were then trapped for another 5 min in adsorbent tubes (Dötterl and Jürgens, 2005; Dötterl et al., 2005) with a 9 V battery-operated pump (Giménez-Benavides et al., 2007). The number of flowers per inflorescence ranged between two and ten, and the age of flowers was 1–4 d. Nine samples (from six plants) were taken during the night (between 2130 and 2315 h) and 11 samples (from the nine plants) during the day (between 0750 and 0930), when most of the flowers were at least partially open. Surrounding air samples were taken as negative controls to distinguish between floral compounds and ambient contaminants. Since we also wanted to assess whether flower VOCs are emitted from the petal limbs or from other parts of the flower, we sampled three plants during the night after removing all the petal limbs (hereafter ‘no petal limbs’ samples). After scent sampling, the flowers in each bag were counted and clipped. To control for the emission of green leaf volatiles (GLVs; Visser et al., 1979; Light et al., 1993), we took one sample from vegetative parts (leaves and stems) and the volatiles detected were deleted from the matrix of flower scent compounds.
The volatiles trapped were analysed by gas chromatogrphy–mass spectrometry (GC-MS) using an automatic thermal desorption (TD) system (TD-20, Shimadzu, Japan) coupled to a Shimadzu GCMS-QP2010 Ultra equipped with a ZB-5 fused silica column (5 % phenyl polysiloxane; 60 m, i.d. 0·25 mm, film thickness 0·25 μm, Phenomenex). The samples were run with a split ratio of 1:1 and a constant helium carrier gas flow of 1·5 mL min–1. The gas chromatograph oven temperature started at 40 °C, then increased by 6 °C min–1 to 250 °C and held for 1 min. The MS interface worked at 250 °C. Mass spectra were taken at 70 eV (EI mode) from m/z 30 to 350. GC-MS data were processed using the GCMSolution package, Version 2·72 (Shimadzu Corporation 2012). Identification of the compounds was carried out using the NIST 11, Wiley 9, FFNSC 2 and Adams (Adams, 2007) databases as well as the database available in MassFinder 3. Some of the compounds were confirmed by comparing mass spectra and retention times with those of synthetic reference compounds. Total scent emission was estimated by injecting known amounts of monoterpenoids, aromatics and aliphatics (added to adsorbent tubes). The mean response of these compounds (mean peak area) was used to determine the total amount of each compound extracted from the adsorbent tubes (Dötterl et al., 2005). For each sample and compound, we calculated the absolute amount emitted (ng) by flower (number) and time (min).
Nectar secretion dynamics
To characterize the temporal variation of nectar production, we took samples from 464 flowers of 23 plants (20 ± 3·1 flowers per plant) available in the insect exclusion cage, from 12 June to 16 July 2013. Before the initiation of anthesis, several cohorts of flower buds were randomly marked with colour codes to control for flower age and sexual stage. Flowers were sampled until 3 d after the beginning of anthesis. Nectar was sampled in three time intervals, morning (1000–1300 h), late afternoon (1700–1900 h) and night (2100–2300 h), with 0·25 μL calibrated microcapillaries (Drummond Scientific Co.). The length of the nectar column was measured with a digital caliper to calculate the extracted volume (μL). The calyx tube of S. colorata is deep and narrow, so the nectar extraction involved opening of this tube. We quantified the nectar accumulated from anthesis to each measurement time (Witt et al., 1999). Sample size was large (n = 30–70 at each time interval) to cope with the intrinsic variation of nectar measurements and with the high frequency of nectarless flowers (Witt et al., 1999).
Anther dehiscence, breeding system and pollination success throughout the day
To assess whether nyctinastic flower opening is coupled with the release of pollen grains and the elongation of the style, we carried out direct observations from initial flower opening until the fourth day of each flower. We observed 2–3 flowers each from five plants in June 2012, and captured a long series of photographs every 15 min with a 90 mm macro lens to make a time-lapse sequence (Bieleski et al., 2000).
To test for variation in pollination success after manual pollen supply throughout the day, we performed a hand pollination experiment on June–July 2012. We randomly assigned 263 flowers from 117 plants (2·2 ± 0·2 flowers per plant) to one of the following time intervals, ‘Morning’ (0900–1100 h) ‘Afternoon’ (1530–1900 h) and ‘Night’ (2100–2300 h). Pollinated flowers were in the second or third day of the female state. Pollen was collected immediately before pollination. All hand pollinations were performed with pollen from another plant of the same population (intra-population xenogamy), assigned randomly.
Additionally, to investigate the breeding system of S. colorata (i.e. its dependence on pollinators), we randomly assigned 296 flower buds from 137 plants (3·0 ± 0·1 buds per plant) to one of the following treatments: (1) spontaneous autogamy (flowers were individually bagged at the bud stage); (2) geitonogamy (hand pollination with pollen from another flower of the same plant); (3) intra-population xenogamy; and (4) inter-population xenogamy, with pollen from a population located >4·5 km away (Table S1). Pollen donors were randomly assigned within treatments. Pollinations were carried out only at night (2100–2300 h) because it was the time when the highest pollination success was achieved in the previous experiment (see the Results).
In both experiments, pollen was supplied by taking one anther of the donor flower with forceps and brushing it on the stigmas of the recipient flower. Flowers were bagged at the bud stage with organza bags (4 × 3 cm), opened for hand pollinations (if appropriate), and re-bagged thereafter until fruit ripening to avoid pollen contamination from other flowers. Flowers were not de-anthered, but the possible self-contamination was controlled with the spontaneous autogamy treatment. After 2–4 weeks, we sampled all fruits to calculate fruit set (proportion of flowers setting fruit) and the number of seeds per fruit. To describe the breeding system, we calculated a modification of the self-incompatibility index (ISI; Zapata and Arroyo, 1978). The ISI was calculated both with fruit set and number of seeds, dividing the success of geitonogamy by the success of intra-population or inter-population xenogamy. Values ≤0·25 indicate self-incompatibility (Sobrevilla and Arroyo, 1982; Faria et al., 2012). Finally, we tried to explore the variation in pollen viability throughout the day by germination tests. Pollen collected at the same time intervals was placed onto Petri dishes containing agar with 30 % sucrose (Buide and Guitán, 2002). However, the culture medium used to test the pollen viability did not work in this species, despite the fact that it was the best medium for a related species, S. acutiflora (Buide and Guitán, 2002).
Flower visitation rates and reproductive success in natural populations
To assess whether the floral phenotype of S. colorata is adapted to a particular type or functional group of pollinators (sensu Fenster et al., 2004), we conducted a field test from 5 to 22 May 2012. Censuses of flower visitors were made in three natural populations (2 Paso, Mostoles and Xanadu), for a total of 15–20 h of observation per population. To collect visitation data, we established five 1 × 1 m sampling plots in each population. The density of plants per plot varied between populations (13·66 ± 2·3, 26·83 ± 6·9 and 22·45 ± 5·89 plants m–2 in 2 Paso, Mostoles and Xanadu, respectively) and the number of flowers per plot ranged between 25 and 320 at the flowering peak. Diurnal observations were made on sunny days without wind, at different time intervals from 1000 to 1900 h. At each time interval, we made observations of 5–10 min in each plot and noted the identity and number of contacts of insect species with the reproductive structures of the flowers. We made 146 censuses, corresponding to 11·8 h of observation. Nocturnal observations were conducted with customized digital video cameras equipped with near-infrared light. On each census date, we placed one camera 15–30 cm in front of each sampling plot and counted the number of open flowers the camera was framing. We recorded each plot continuously from 2100 to 0100 h. We visualized a total of 40·1 h to note the identity and frequency of flower visitors. We could not accurately distinguish among moths species in the night video records. All visiting insects touching sexual organs were considered pollinators regardless of the efficacy of the visit. Insects were grouped into functional groups to calculate visitation rates (visits per flower h–1) per time interval (morning, 0900–1500 h; afternoon, 15:00–1900 h; and night, 2100–0100 h). The minutes of observation per population ranged between 150 and 200 in the morning, between 50 and 100 in the afternoon and between 672 and 1078 in the night.
Two to three weeks after pollination census (25 May–4 June 2012), we randomly sampled ten plant individuals that had completed the full life cycle in each plot. We counted the total number of flowers (dried or aborted) and fruits produced per plant to calculate the natural fruit set in the populations. The rate of fruit predation (number of predated fruits/total number of fruits) by the Hadena nursery pollinators was also estimated because the larvae of these moths leave a characteristic hole in the capsules (pers. obs). Non-predated fruits were dissected to count the number of seeds. Outside the plots, we also collected green fruits that were carried to the laboratory. After some days, the Hadena larvae that emerged from the parasitized fruits thereof were reared until the adult stage to identify the species.
Statistical analysis
To explore the effect of light intensity and soil moisture on the dynamic of flower opening, a LMM (linear mixed model) was carried out with the following explanatory variables: light (Shade and Sun), moisture (Wet and Dry), time (every 30 min) and the interaction light × moisture × time. These factors were computed as fixed effects. Flower and plant were computed as random effects. The percentage of petal opening was arcsin [square root(X)] transformed before analysis to achieve normality. To explore the nectar dynamics, another LMM was applied with day (first, second and third), time of sampling (morning, late afternoon and night) and the interaction day × time of sampling as the explanatory variables. Nectar volume was square root transformed to achieve normality. These factors were computed as fixed effects and plant as random effect.
To analyse the variation of flower scent depending on the treatment (morning, night and flowers without petals), a set of generalized linear model (GLM) analyses were made for total scent production and the production of each compound independently. For these GLMs we used a tweedie error structure because of the zero-inflated distribution of the data (Dunn and Smyth, 2005; Tascheri et al., 2010). To depict variation in floral scent composition among samples, we used non-metric multidimensional scaling (NMDS). To test the differences in the complete scent (relative scent composition) among the treatments, a permutational multivariate analysis of variance (PERMANOVA) was made. A Bray–Curtis pairwise matrix of similarities (Clarke and Gorley, 2006) based on the percentage amount of the compounds was used for NMDS and PERMANOVA. To avoid that NMDS and PERMANOVA were greatly influenced by the most abundant compounds, the data (percentage contribution of the compounds to the total scent) were fourth root transformed (Clark and Warwick, 2001). To evaluate the breeding system, and the differences in pollination success throughout the day, we used generalized linear mixed models (GLMMs) for fruit set and LMMs for number of seeds. Treatment (geitonogamy, intra-population xenogamy and inter-population xenogamy) and pollination time (morning, afternoon and night) were used as explanatory factors for breeding system and pollination success, respectively, and plant identity was computed as a random effect. For fruit set GLMMs, we used Binomial error structure for the presence or absence of fruits (1 when a flower set fruit; 0 when did not). Autogamic hand crossings were excluded from the analyses because they did not produce any fruit (see the Results). To explore differences in flower visitation rate, we carried out a GLM with population, time and population × time. Fruit set, fruit predation (LM) and seed number (GLM) comparisons between populations were performed with population as the explanatory factor. To test the effect of the visitation rate on the pollination success, we performed four (total, morning, afternoon and night rates) LM and four GLM regressions for fruit set and seed number, respectively. For these GLM regressions we used Poisson error structure because the seed number had positive integer values. Post-hoc analyses were performed with the Tukey HSD test. Analysis were implemented with the ‘tweedie’, ‘nlme’, ‘lme4’, ‘car’ and ‘argricolae’ packages (Fox and Weisberg, 2011; Pinheiro et al., 2013; Dunn, 2014; Mendiburu, 2014; Bates et al., 2015) in R software (R Core Team, 2014), except the NMDS and PERMANOVA that were implemented in PRIMER 6·1·11 (Clarke and Gorley, 2006).
RESULTS
Effect of light intensity and soil moisture on flower nyctinasty
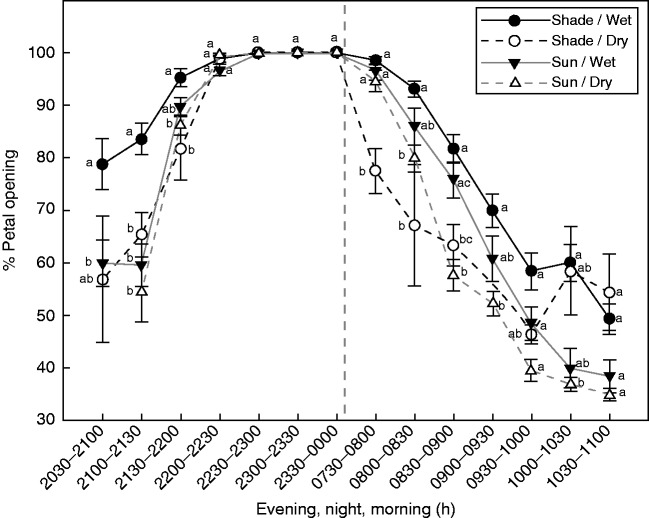
There were significant effects of light intensity (F1,24 = 8·73, P = 0·007) and time of measurement (F13,1525 = 507·9, P < 0·001) on the percentage of petal opening. Soil moisture (F1,24 = 4·13, P = 0·053) was marginally significant, and the interaction moisture × light × time was significant (F13,1525 = 4·01, P < 0·001). In the evening at 2100–2130 h, petals of the Shade–Wet treatment were more unrolled than petals of other treatments (Fig. 1). However, all treatments reached 100 % opening at 2200–2230 h. Petals of all treatments remained open until dawn. In the morning, petals of the Shade–Dry treatment were the first to close at 0730–0800 h, followed by plants under the Sun–Dry conditions at 0830–0900 h. Plants under the Shade–Wet treatment maintained the petals more open than those under Dry treatments until 1000–1030 h. The most fully closed petals at the end of the morning were those under Sun treatments, although differences were not significant.
Fig. 1.
Dynamic of flower opening and closure throughout the day (mean ± s.e. of percentage petal opening) of plants subjected to four combinations of light intensity and soil moisture. Different letters indicate significant differences among treatments during the same time period. The vertical dashed line denotes the transition from night to morning.
Dynamic of flower scent emission
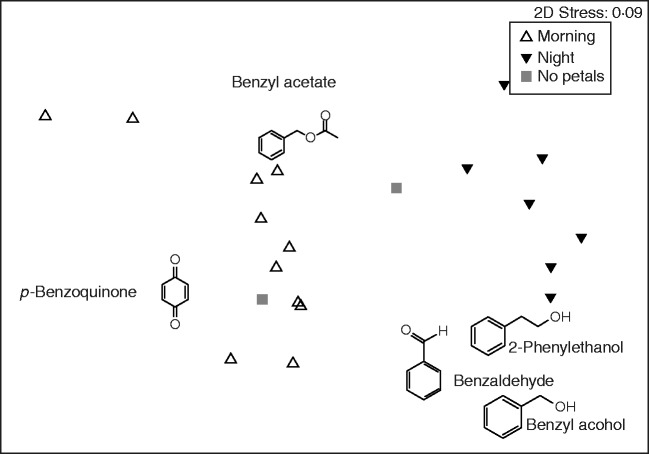
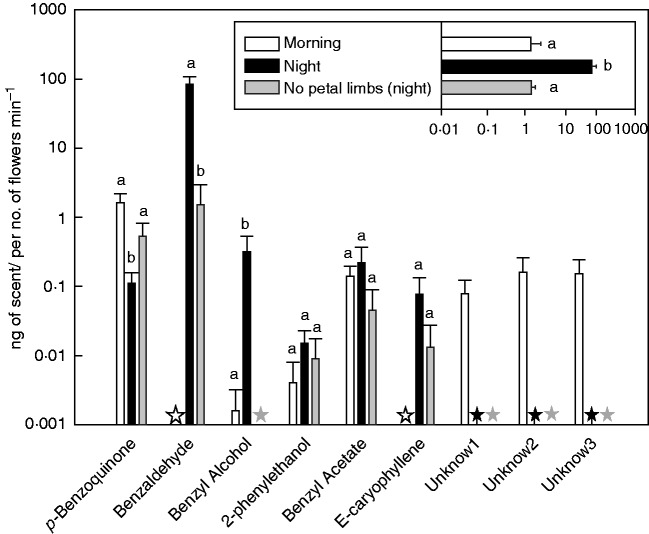
The GC-MS analyses showed that two of the night samples did not emit any scent. These plants had flowers with petals not completely open when volatiles were trapped (2110 h). These two samples were not taken into account in the GLM analysis. Another night sample with the petals excised also did not emit scent. These three samples were not taken into account in the NMDS analysis and PERMANOVA. The scent composition differed among the treatments (pseudo F1,17 = 8·48; P < 0·001). Night samples had a different composition from morning samples and flowers with no petals limbs. There was no difference between morning samples and the scent of flowers with no petal limbs (Fig. 2). The standardized total scent production (ng flower min–1) was higher in the night than in the day and no petal limbs samples (F2,18 = 48·62 P < 0·001) (Fig. 3). There were significant differences among treatments (morning, night and flowers with no petal limbs) with any production in some compounds (benzaldehyde F1,8 = 9·57 P = 0·015; benzyl alcohol F1,16 = 16·99 P < 0·001; p-benzoquinone F2,18 = 6·23 P = 0·009), but not in 2-phenylethanol (F2,18 = 0·74 P = 0·49), benzyl acetate (F2,18 = 0·67 P = 0·52) and E-caryophyllene (F1,8 = 0·9 P = 0·37) (Fig. 3). Benzylaldehyde and E-caryophyllene were emitted in high amounts at night but not during the day. The emission of benzyl alcohol was higher at night than in the day. Conversely, three unknown compounds were only emitted in the morning, and the emission of p-benzoquinone was higher in the day than at night. The excision of the petal limbs reduced the emission of benzaldehyde and eradicated the emission of benzyl alcohol, but increased the amount of p-benzoquinone, and did not affect E-caryophyllene, 2-phenylethanol and benzyl acetate (Fig. 3).
Fig. 2.
Non-metric multidimensional scaling (NMDS) of the flower scent released by S. colorata in the morning (0750–0930 h), at night (2130–2315 h) and at night with petal limbs excised. The NMDS is based on the percentage amount of the compounds. Main volatile compounds are placed following correlations of each compound with the ordination axis.
Fig. 3.
Mean ± s.e. of floral emission rates of each volatile compound. The insert denotes the total floral scent emission rates. Stars denote no production of a compound. Different letters indicate significant differences among treatments. Both graphs are in log scale.
Anther dehiscence, breeding system and pollination success throughout the day
Anther dehiscence of S. colorata took place immediately after the flower bud burst. The two whorls of five stamens dehisced sequentially on the first and second night, and they withered at dusk each. The style elongation began at dusk of the third day. Both anther dehiscence and style elongation are synchronous with nyctinastic flower opening (Supplementary Data Video S1). The pollen was of fresh and dusty appearance from anther dehiscence until mid-day, but then it turned dry and clumpy (pers. obs.).
In the breeding system experiment, the spontaneous autogamy treatment did not produce any fruit. There were no differences between geitonogamy, intrapopulation xenogamy and interpopulation xenogamy in fruit set (χ22 = 5·2 P = 0·07) and in the number of seeds (F2,156 = 0·7 P = 0·5) (Table 1). The ISI values indicated that S. colorata is self-compatible (fruit set ISI = 0·86 and seed number ISI = 0·91 for interpopulation xenogamy; fruit set ISI = 1·01 and seed set ISI = 0·95 for intrapopulation xenogamy). The number of seeds was lower in the afternoon than in the morning and night pollinations (F2,144 = 21·96, P < 0·001, Table 1). Fruit set was lower in the afternoon than in the morning, but fruit set at night was no different from that during the morning and afternoon (χ22 = 9·08, P < 0·012, Table 1).
Table 1.
Number of seeds per fruit and fruit set in the breeding system experiment and the experiment on the pollination success throughout the day
| No. of seeds (mean ± s.d.) | Fruit set (%) | |
|---|---|---|
| Breeding system | ||
| Geitonogamy | 37·03 ± 2·8 | 77 |
| Intra-population xenogamy | 38·95 ± 2·2 | 87 |
| Inter-population xenogamy | 40·64 ± 4·0 | 75 |
| Pollination success | ||
| Night | 39 ± 2·2a | 87ab |
| Morning | 44·8 ± 2·3a | 93a |
| Afternoon | 18·4 ± 3·0b | 74b |
Different letters indicate significant differences among treatments.
Nectar dynamic
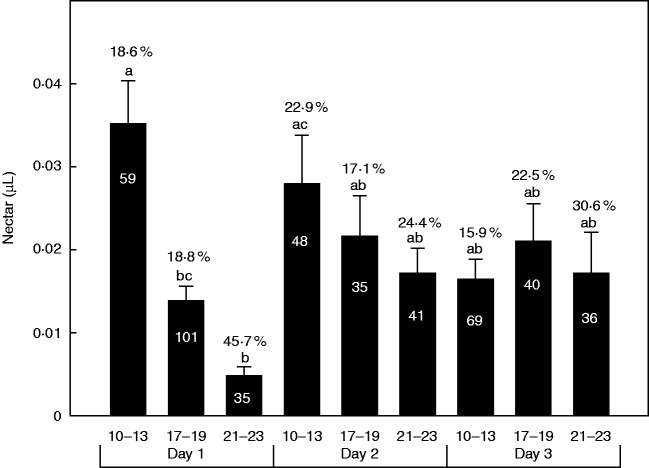
Nectar production was very low in S. colorata (range = 0–0·21 μL) and there was a high proportion of flowers that did not produce nectar (Fig. 4). Nonetheless, there were significant differences in nectar volume between time intervals (F2,433 = 5·05, P = 0·007) and in the interaction day × time of sampling (F4,433 = 3·1, P = 0·02) but not between days (F2,455 = 2·61, P = 0·07). The first morning after flower opening, nectar volume was high, and then decreased during the course of the day (Fig. 4). On the second day, flowers showed the same pattern but there were no significant differences among times of the day. On the third day (first in the female stage) there were also no significant differences (Fig. 4).
Fig. 4.
Each bar represent the mean nectar volume (μL) accumulated from anthesis to the time of measurement (h) in flowers of S. colorata. Note that the first bar corresponds to the morning after flower bud opening at dusk. On day 1 and 2, flowers are in the male stage; on day 3 flowers reach the female stage. Error bars denote the s.e. Different letters indicate significant differences among time intervals. Numbers above each bar denote the percentage of nectarless flowers. The number within each bar denotes the sample size.
Flower visitation rates, reproductive success and fruit predation by the nursery pollinator
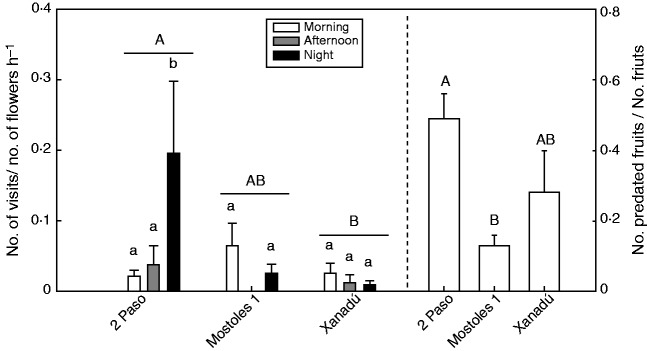
Field results showed that S. colorata was visited by both diurnal and nocturnal insects (Fig. 5). In the daytime (morning and afternoon), small bees were the most frequent visitors (range from 81 to 100 % of diurnal visits), followed by bombilid flies (0–18 %) and hoverflies (0–1 %). At night, all visitors were moths (Noctuidae and Geometridae). The visitation rate varied between time intervals (F2,43 = 5·50, P = 0·008), and between populations (F2,43 = 7·85, P = 0·002) (Fig. 5). Also there was a significant effect of the interaction between population and time interval (F4,43 = 5·26, P = 0·002) (Fig. 5). Fruit set and number of seeds were not different among populations (F2,12 = 0·41, P = 0·67 and F2,12 = 0·59, P = 0·57, respectively). Fruit set was not affected by visitation rates (F1,13 = 0·67, P = 0·43; F1,13 = 0·02, P = 0·89; F1,13 = 1·59, P = 0·23; F1,12 = 2·45, P = 0·14; for the total, morning, afternoon and night visitation rate, respectively). There was a positive influence of the total (Z1,12 = 4·04, P = 0·04), night (Z1,12 = 2·16, P = 0·031) and afternoon (Z1,11 = 2·03, P = 0·042) visitation rate on the seed number, but no effect of the morning visitation rate (Z1,11 = –1·84, P = 0·14). There were differences among populations on the fruit predation rate by Hadena sancta (F2,11 = 4·51, P = 0·037) (Fig. 5), the only species that emerged from fruits of S. colorata (n = 23). The fruit predation rate was higher at the 2 Paso population, which also had the highest number of nocturnal visits (Fig. 5).
Fig. 5.
Variation among S. colorata populations in flower visitation rates at each time interval (left panel), and fruit predation rate (right panel). Vertical bars denote means ± s.e. Different upper case letters indicate significant differences among populations. Different lower case letters denote differences among time intervals within each population.
DISCUSSION
Flower traits and nyctinasty
The first observation of the floral phenotype of S. colorata is an interesting contradiction. The species has bright pink petals which points towards a diurnal pollination system (Jürgens, 2006), but flowers are fully opened only at night. Flower colour has been one of the classical features for definition of pollination syndromes (Faegri and van der Pijl, 1979; Proctor et al., 1996), but it must be treated with caution as a key trait (Waser et al., 1996). In Sileneae, the flower colour is not very reliable as a predictor trait for diurnal or nocturnal pollination (Prieto-Benítez et al., 2015).
Our results suggest that the nyctinastic petal folding in S. colorata is influenced by changes in light intensity and accelerated or delayed by soil water content. In shadow microhabitats (e.g. beneath trees or shrubs), the light intensity decreases earlier at dusk and increases later at dawn, and the soil retains more water. In consequence, the flowers open earlier and close later, extending the period of flower display, resulting in an increase in the visibility for evening and early morning flower visitors. In sunny and dry microhabitats, petal closure is accelerated at dawn and the petals remain closed (rolled) during the whole day, reducing the temporal range of floral display. The closing and opening of petals was also influenced by light intensity and air humidity in Silene saxifraga (Halket, 1931). Halket showed in this pioneer work that closing of petals is due to the loss of cell water content by transpiration, and opening is due to cell refilling probably in response to a combination of sugar uptake and degradation of polysaccharides (van Doorn and van Meeteren, 2003). The maintenance of turgor in the petals requires a constant input of water from vegetative parts (Ram and Rao, 1984), and this demand may involve a high cost especially in dry environments (Nobel, 1977; Galen et al., 1999; Teixido and Valladares, 2013, 2014). Since light is the most important input signal to the circadian clock (McClung, 2006; Sanchez et al., 2011), an increase in light intensity may regulate the flower closure to signal to the plant the high evapotranspirational demand in the middle hours of the day. Minimizing floral water loss by nocturnal flowering may be an advantageous strategy in hot and dry ecosystems (Teixido and Valladares, 2014), and is found in other Mediterranean plants such as Capparis species (Rhizopoulou et al., 2006), and in most desert cacti (Valiente-Banuet et al., 1997; Fleming et al., 2001).
The significance of flower closure response to the abiotic environment may be considerable for several reasons. First, plastic responses of floral attractiveness to the environmental conditions may influence the suite of pollinators and how they forage flowers during the day and season, affecting the plant fitness. This is important because the actual climate change is affecting the abiotic factors regulating floral attractiveness. Secondly, if plant genotypes vary in their sensitivity to abiotic factors, some of the variation in flower opening and closure among individuals may be caused by heritable differences, and this may be subject to selection. Moreover, selection of flower traits imposed by the abiotic environment and by pollinators may conflict (Carroll et al., 2011). The maintenance of flower nyctinasty may be constrained by a trade-off between water economy and attractiveness to diurnal pollinators (despite the fact that morning pollinators may select for longer periods of flower opening, drought stress may favour genotypes with rapid flower closure).
Nectar secretion, flower scent and sexual phases
We have found that nyctinastic petal opening is synchronized with nectar secretion dynamics in S. colorata. Nectar production took place only at night, at least on the first and second day after anthesis. On the third day (first day of the female phase), this pattern was lost. Since insect visits were excluded in our experiment, the reduction in nectar volume from night to afternoon could be due to evaporation or resorption. Evaporation of nectar may be limited in this species due to the high osmolarity provided by the high hexose (glucose and fructose) content (Witt et al., 2013) and the availability of nectar within a long floral tube, which might reduce the evaporative effects of a low relative humidity of the air (Pacini and Nepi, 2007). However, nectar resorption is a widespread strategy in unvisited flowers, presumably to recover the resources invested in nectar production (Burquez and Corbet, 1991; Pacini and Nepi, 2007). Nocturnal nectar production also occurs in other Silene species with nocturnal pollination syndrome (Witt et al., 1999; Reynolds et al., 2009; Castillo et al., 2013), and similar diel patterns have been previously described in other species pollinated by nocturnally active animals (Cruden et al., 1983; Tschapka and von Helversen, 2007; Amorim et al., 2013). The high number of nectarless flowers in this species is in accordance with previous findings in several plant species and also in Silene (Brink, 1982; May, 1988; Gilbert et al., 1991; Witt et al., 1999).
Nyctinastic flower opening and nectar secretion are also synchronized with the emission of high amounts of scent at night. The petals usually produce most of the VOCs of the flower scent (Dobson et al., 1990; Bergström et al., 1995). When petal limbs were abscised at night, scent amount and composition decreased significantly, and was similar to those of intact flowers at morning. This suggests that emission of the most abundant flower VOCs at night, benzaldehyde and benzyl alcohol, takes place mainly in the expanded petals, whereas other compounds (i.e. p-benzoquinone, E-caryyophyllene, 2-phenylethanol and benzyl acetate) are released from other floral organs. Petals are also responsible of the benzenoid emission in S. latifolia (Dötterl and Jürgens, 2005) and are also the main scent producer in Petunia and Antirrhinum flowers (Dudareva et al., 2000b; Verdonk et al., 2003). When petals are folding in the morning, the scent production ceases, and in the afternoon S. colorata do not produce any flower scent (Prieto-Benítez et al., 2015) until the petals open again at night.
Anther dehiscence and style elongation were also synchronized with petal opening. Anther dehiscence at dusk may provide a fresh atmosphere for pollen during the night, but in the morning temperature increases and the pollen grains get dry and clump together (pers. obs.), decreasing their viability (Nepi et al., 2001). In the nocturnal S. latifolia, in vitro pollen germinability reaches the maximum at midnight and then decreases (Aonuma et al., 2013). In contrast, in the diurnal S. acutifolia, pollen germinability declined progressively after dehiscence in the daytime (Buide and Guitán, 2002). Unfortunately the culture medium used by these authors did not work in S. colorata, so we cannot prove the loss of viability in the daytime. In any case, our experiment showed that hand pollination yielded higher reproductive success (both fruit set and number of seeds) at night and in the morning than in the afternoon. These differences may indeed be due to the reduction of pollen viability, but may also be due to a decrease in stigma receptivity, since it is known that high temperatures at mid-day can reduce stigmatic receptivity (Hedhly et al., 2005).
Breeding system, pollinators and predators
Silene colorata is self-compatible as are many other Silene species (Bocquet, 1968). Spontaneous autogamy is not viable due to protandry (the anthers mature and wilt before the elongation of styles), so this species depends on flower visitors even for geitonogamous pollination. The opening of petals at dusk may attract moths visually and additionally provide a landing platform for settling moths. At the same time, the production of nectar reward increases in parallel with the emission of high amounts of benzaldehyde, benzyl alcohol, 2-phenylethanol and benzyl acetate, all of them related to the attraction of moths (Heath et al., 1992; Meagher, 2002; Dobson, 2006; Giménez-Benavides et al., 2007). These flower VOCs are also present in the nocturnal scent of other Silene species with moth pollinators, such as S. subconica, S. viscosa, S. latifolia and S. ciliata (Jürgens et al., 2002; Dötterl et al., 2005; Giménez-Benavides et al., 2007; Castillo et al., 2014). This combination of traits may lead the moths to remove the fresh pollen just after dehiscence and to deposit it on the young receptive styles, increasing their efficiency as pollinators.
In the morning, other insects such as bees, bombilids and hoverflies also visit S. colorata and may pollinate the flowers with the remaining pollen. The interplay of colour and scent is essential for diurnal insects for finding and recognizing host-plants (Burger et al., 2010; Milet-Pinheiro et al., 2012). Pink petals are attractive to bees (Menzel and Shmida, 1993; Reynolds et al., 2009), so they can be a visual cue of S. colorata early in the morning, when they are still open, and even when they are completely rolled up in the afternoon. Although we previously found that S. colorata did not emit flower VOCs at mid-day (Prieto-Benítez et al., 2015), the flowers still emit a small amount of scent before complete petal closure, which differs in composition from the nocturnal scent. Among the compounds released during the day, p-benzoquinone, benzyl acetate, benzyl alcohol and 2-phenylethanol have the potential to attract bees and flies (Knudsen and Mori, 1996; Dobson, 2006; Dötterl and Vereecken, 2010; Burger et al., 2012). It is also interesting to note that three unknown compounds are emitted in significant amounts only in the morning. These compounds may act as olfactory cues for diurnal attraction of pollinators or as deterrents of herbivores. Small bees and hoverflies visited the flowers of S. colorata to collect pollen, and bombilids to drink nectar. Both rewards are less abundant but still available during the day, especially in the early morning, if they have not been consumed the previous night. Moreover, our hand pollination experiment showed that one pollen grain has the same probability to develop a seed when it is deposited in a flower at night as in the early morning, but pollination success decreases by 2-fold in the afternoon. This suggests that phenotypic specialization to night pollination in S. colorata does not cause a strong fitness trade-off to early morning pollination, so the functional diversity of pollinators can be maintained (Aigner, 2001, 2004; Armbruster, 2014). However, in the afternoon, the attractiveness and fertility of flowers reduce drastically, and this may result in a large decrease in fertilization success.
Our pollinator censuses showed that the abundance of day and night visitors varies between the three populations (Fig. 5), and moths were the most frequent visitors only in one population. The number of seeds per fruit was positively correlated with night, afternoon and total visit rates. The number of seeds was not correlated with the morning visit rate, despite the fact that our hand pollination experiment found that morning pollination can produce high amounts of seeds. This apparent contradiction could be due to a low efficiency (pollen removed and deposited per single visit) of the morning pollinators. We did not collect efficiency data, but Reynolds et al. (2009) reported that pollinator importance (visitation frequency × pollen deposition) was higher for nocturnal moths than for diurnal bees in S. stellata (another species with nocturnal moth syndrome) in two of three studied years. In summary, the results of hand pollinations and field censuses together suggest that when nocturnal pollinators are scarce, the combined effect of diurnal and nocturnal pollination may ensure the plant’s reproductive success. Complementarity of diurnal and nocturnal pollination has been described before (Miyake and Yahara, 1999; Wolff et al., 2003; Reynolds et al., 2009; Amorim et al., 2013). We believe that our observations on the flower circadian rhythm and receptivity of S. colorata may be generalized to other Silene species with presumed nocturnal syndrome. Many of them have their flowers open in the early morning (pers. obs.), and diurnal pollinators are also frequent and have a substantial role in their reproductive success (Giménez-Benavides et al., 2007; van Putten et al., 2007; Reynolds et al., 2009; Martinell et al., 2010; Buide et al., 2015). These results suggest functional reasons to support the general agreement that traits associated with classical pollination syndromes can vary and directly impact the pollinators observed in the field (Waser et al., 1996; Ollerton et al., 2007, 2015; Armbruster, 2014).
The presence of the nursery pollinator Hadena sancta in the nocturnal pollinator guild of S. colorata, which is for the first time described as a host in the present work, may explain why there was no positive effect of the visitation rate on the fruit set. Hadena sancta pollinates the plant but also rears its offspring in the flowers and developing fruits. Although the pollinator service provided by Hadena species may be prominent (Reynolds et al., 2009, 2012; Labouche and Bernasconi, 2010), the net effect of the interaction may be negative when the cost of fruit predation is taken into account (Kephart et al., 2006; Reynolds et al., 2012; Kula et al., 2014). Unfortunately, we could not accurately distinguish Hadena from other moths in the night video recordings to estimate its visitation frequency. However, the overall nocturnal visitation rate was positively correlated with fruit predation by Hadena larvae in two out of three populations (2 Paso and Mostoles) (Fig. 5), suggesting that loss by fruit predation is proportional to pollination service in these two populations. If the net outcome of the S. colorata–H. sancta interaction shifts towards parasitism, the complementary pollination provided by diurnal pollinators may help to compensate the high cost of fruit predation. The outcome of nursery pollination has been studied in other Silene–Hadena systems (Petersson, 1991; Reynolds et al., 2012; Kula et al., 2014). These works have shown that the frequency of the nursery pollinators and co-pollinators contributes to shifts between mutualism and parasitism with the host plant, and this outcome also varies in space and time and with host plant density. Selective pressures exerted by pollinators and predators may also vary in space and time (Thompson, 1994, 1999) and in trait combinations. For instance, Fenster et al. (2015) demonstrated complex selection by hummingbirds in artificial combinations of flower traits from three contrasting Silene species. Therefore, studies that involve several populations and various years are needed to clarify the constancy or lability of selective pressures acting on floral traits of S. colorata.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Table S1: localities from where the seed came and the data of the flower visitor census. Video S1: petal nyctinasty, anther dehiscence and style elongation of Silene colorata.
ACKNOWLEDGEMENTS
We would like to thank J. L. Margalet for caring for the plants. This work was supported by the MINECO research project of the Spanish Government [CGL2009–08755].
LITERATURE CITED
- Adams RP. 2007. Identification of essential oil components by gas chromatography/mass spectrometry. Carol Stream, IL: Allured. [Google Scholar]
- Aigner PA. 2001. Optimality modeling and fitness trade-offs: when should plants become pollinator specialists? Oikos 95: 177–184. [Google Scholar]
- Aigner PA. 2004. Floral specialization without trade-offs: optimal corolla flare in contrasting pollination environments. Ecology 85: 2560–2569. [Google Scholar]
- Amorim FW, Galetto L, Sazima M. 2013. Beyond the pollination syndrome: nectar ecology and the role of diurnal and nocturnal pollinators in the reproductive success of Inga sessilis (Fabaceae). Plant Biology 15: 317–327. [DOI] [PubMed] [Google Scholar]
- Aonuma W, Shimizu Y, Ishii K, Fujita N, Kawano S. 2013. Maturation timing of stamens and pistils in the dioecious plant Silene latifolia. Journal of Plant Research 126: 105–112. [DOI] [PubMed] [Google Scholar]
- Armbruster WS. 2014. Floral specialization and angiosperm diversity: phenotypic divergence, fitness trade-offs and realized pollination accuracy. AoB PLANTS 6: plu003. doi:10.1093/aobpla/plu003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassani M, Pacini E, Franchi GG. 1994. Humidity stress responses in pollen of anemophilous and entomophilous species. Grana 33: 146–150. [Google Scholar]
- Bates D, Maechler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67: 1–48. [Google Scholar]
- Bernasconi G, Antonovics J, Biere A, et al. 2009. Silene as a model system in ecology and evolution. Heredity 103: 5–14. [DOI] [PubMed] [Google Scholar]
- Bergström G, Dobson HE, Groth I. 1995. Spatial fragrance patterns within the flowers of Ranunculus acris (Ranunculaceae). Plant Systematics and Evolution 195: 221–242. [Google Scholar]
- Bieleski R, Elgar J, Heyes J, Woolf A. 2000. Flower opening in Asiatic lily is a rapid process controlled by dark–light cycling. Annals of Botany 86: 1169–1174. [Google Scholar]
- Bocquet G. 1968. Physolychnidum morphologica catalecta. Candollea 23: 151–176. [Google Scholar]
- Buide ML, Guitián J. 2002. Breeding system in the dichogamous hermaphrodite Silene acutifolia (Caryophyllaceae). Annals of Botany 90: 691–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buide ML, del Valle JC, Pissatto M, Narbona E. 2015. Night life on the beach: selfing to avoid pollinator competition between two sympatric Silene species. Annals of Botany 116: 201–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Búrquez A, Corbet SA. 1991. Do flowers reabsorb nectar? Functional Ecology 5: 369–379. [Google Scholar]
- Burger H, Dötterl S, Ayasse M. 2010. Host-plant finding and recognition by visual and olfactory floral cues in an oligolectic bee. Functional Ecology 24: 1234–1240. [Google Scholar]
- Burger H, Dötterl S, Häberlein CM, Schulz S, Ayasse M. 2012. An arthropod deterrent attracts specialised bees to their host plants. Oecologia 168: 727–736. [DOI] [PubMed] [Google Scholar]
- Brink D. 1982. A bonanza–blank pollinator reward schedule in Delphinium nelsonii (Ranunculaceae). Oecologia 52: 292–294. [DOI] [PubMed] [Google Scholar]
- Carroll AB, Pallardy SG, Galen C. 2001. Drought stress, plant water status, and floral trait expression in fireweed, Epilobium angustifolium (Onagraceae). American Journal of Botany 88: 438–446. [PubMed] [Google Scholar]
- Castillo DM, Kula AA, Fenster KA, Fenster CB, Dudash MR. 2013. Specialist pollinating seed predator exhibits oviposition strategy consistent with optimal oviposition theory. Ecological Entomology 38: 164–172. [Google Scholar]
- Castillo DM, Kula AA, Dötterl S, Dudash MR, Fenster CB. 2014. Invasive Silene latifolia may benefit from a native pollinating seed predator, Hadena ectypa, in North America. International Journal of Plant Sciences 175: 80–91. [Google Scholar]
- Clarke KR, Gorley RN. 2006. PRIMER v6: user manual/tutorial. PRIMER-E, Plymouth-E Ltd. [Google Scholar]
- Clarke KR, Warwick RM. 2001. Change in marine communities: an approach to statistical analysis and interpretation. PRIMER-E, Plymouth-E Ltd. [Google Scholar]
- Cruden RW, Hermann SM, Peterson S. 1983. Patterns of nectar production and plant–pollinator coevolution In: Bentley B, Elias TS, eds. The biology of nectarines. New York: Columbia University Press, 80–125. [Google Scholar]
- Darwin C, Darwin F. 1880. The power of movement in plants. London: John Murray. [Google Scholar]
- Dobson HE. 2006. Relationship between floral fragrance composition and type of pollinator In: Dudareva N, Pichersky E, eds. Biology of floral scent. Boca Raton, FL: Taylor & Francis Group, 147–198. [Google Scholar]
- Dobson HE, Bergström G, Groth I. 1990. Differences in fragrance chemistry between flower parts of Rosa rugosa Thunb. (Rosaceae). Israel Journal of Botany 39: 143–156. [Google Scholar]
- van Doorn WG, van Meeteren U. 2003. Flower opening and closure: a review. Journal of Experimental Botany 54: 1801–1812. [DOI] [PubMed] [Google Scholar]
- Dötterl S, Jürgens A. 2005. Spatial fragrance patterns in flowers of Silene latifolia: lilac compounds as olfactory nectar guides? Plant Systematics and Evolution 255: 99–109. [Google Scholar]
- Dötterl S, Vereecken NJ. 2010. The chemical ecology and evolution of bee–flower interactions: a review and perspective. Canadian Journal of Zoology 88: 668–697. [Google Scholar]
- Dötterl S, Wolfe LM, Jürgens A. 2005. Qualitative and quantitative analyses of flower scent in Silene latifolia. Phytochemistry 66: 203–213. [DOI] [PubMed] [Google Scholar]
- Dudareva N, Piechulla B, Pichersky E. 2000a. Biogenesis of floral scents. Horticultural Reviews 24: 31–53. [Google Scholar]
- Dudareva N, Murfitt LM, Mann CJ, et al. 2000b. Developmental regulation of methyl benzoate biosynthesis and emission in snapdragon flowers. The Plant Cell 12: 949–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn PK. 2014. tweedie: Tweedie exponential family models. R package version 2.2.1.
- Dunn PK, Smyth GK. 2005. Series evaluation of Tweedie exponential dispersion model densities. Statistics and Computing 15: 267–280. [Google Scholar]
- Faegri K, van der Pijl 1979. The principles of pollination ecology. Oxford: Pergamon. [Google Scholar]
- Faria RR, Ferrero V, Navarro L, Araujo AC. 2012. Flexible mating system in distylous populations of Psychotria carthagenensis Jacq. (Rubiaceae) in Brazilian Cerrado. Plant Systematics and Evolution 298: 619–627. [Google Scholar]
- Fenster CB, Armbruster WS, Wilson P, Dudash MR, Thomson JD. 2004. Pollination syndromes and floral specialization. Annual Review of Ecology, Evolution, and Systematics 35: 375–403. [Google Scholar]
- Fenster CB, Reynolds RJ, Williams CW, Makowsky R, Dudash MR. 2015. Quantifying hummingbird preference for floral trait combinations: the role of selection on trait interactions in the evolution of pollination syndromes. Evolution 69: 1113–1127. [DOI] [PubMed] [Google Scholar]
- Fleming TH, Sahley CT, Holland JN, Nason JD, Hamrick JL. 2001. Sonoran Desert columnar cacti and the evolution of generalized pollination systems. Ecological Monographs 71: 511–530. [Google Scholar]
- Fox J, Weisberg S. 2011. An {R} companion to applied regression, 2nd edn Thousand Oaks, CA: Sage. [Google Scholar]
- Galen C, Newport MEA. 1987. Bumble bee behavior and selection on flower size in the sky pilot, Polemonium viscosum. Oecologia 74: 20–23. [DOI] [PubMed] [Google Scholar]
- Galen C, Sherry RA, Carroll AB. 1999. Are flowers physiological sinks or faucets? Costs and correlates of water use by flowers of Polemonium viscosum. Oecologia 118: 461–470. [DOI] [PubMed] [Google Scholar]
- Gilbert FS, Haines N, Dickson K. 1991. Empty flowers. Functional Ecology 5: 29–39. [Google Scholar]
- Giménez-Benavides L, Dötterl S, Jürgens A, Escudero A, Iriondo JM. 2007. Generalist diurnal pollination provides greater fitness in a plant with nocturnal pollination syndrome: assessing the effects of a Silene–Hadena interaction. Oikos 116: 1461–1472. [Google Scholar]
- Gómez JM, Zamora R. 2006. Ecological factors that promote the evolution of generalization in pollination systems In: Waser NM, Ollerton J, eds. Plant–pollinator interactions: from specialization to generalization. Chicago, IL: University of Chicago Press, 145–166 [Google Scholar]
- Greuter W. 1995. Silene (Caryophyllaceae) in Greece: a subgeneric and sectional classification. Taxon 44: 543–581. [Google Scholar]
- Halket AC. 1931. The flowers of Silene saxifraga, L.; an inquiry into the cause of their day closure and the mechanism concerned in effecting their periodic movements. Annals of Botany 1: 15–36. [Google Scholar]
- Heath RR, Landolt PJ, Dueben B, Lenczewski B. 1992. Identification of floral compounds of night-blooming jessamine attractive to cabbage looper moths. Environmental Entomology 21: 854–859. [Google Scholar]
- Hedhly A, Hormaza JI, Herrero M. 2005. The effect of temperature on pollen germination, pollen tube growth, and stigmatic receptivity in peach. Plant Biology 7: 476–483. [DOI] [PubMed] [Google Scholar]
- Herrera CM. 1996. Floral traits and plant adaptation to insect pollinators: a devil’s advocate approach In: Lloyd D, Barrett S, eds. Floral biology. Springer; US, 65–87. [Google Scholar]
- Hurlbert AH, Hosoi SA, Temeles EJ, Ewald PW. 1996. Mobility of Impatiens capensis flowers: effect on pollen deposition and hummingbird foraging. Oecologia 105: 243–246. [DOI] [PubMed] [Google Scholar]
- Johnson SD, Steiner KE. 2000. Generalization versus specialization in plant pollination systems. Trends in Ecology and Evolution 15: 140–143. [DOI] [PubMed] [Google Scholar]
- Jürgens A. 2004. Flower scent composition in diurnal Silene species (Caryophyllaceae): phylogenetic constraints or adaption to flower visitors? Biochemical Systematics and Ecology 32: 841–859. [Google Scholar]
- Jürgens A. 2006. Comparative floral morphometrics in day-flowering, night-flowering and self-pollinated Caryophylloideae (Agrostemma, Dianthus, Saponaria, Silene, and Vaccaria). Plant Systematics and Evolution 257: 233–250. [Google Scholar]
- Jürgens A, Witt T, Gottsberger G. 2002. Flower scent composition in night-flowering Silene species (Caryophyllaceae). Biochemical Systematics and Ecology 30: 383–397. [Google Scholar]
- Kephart S, Reynolds RJ, Rutter MT, Fenster CB, Dudash MR. 2006. Pollination and seed predation by moths on Silene and allied Caryophyllaceae: evaluating a model system to study the evolution of mutualisms. New Phytologist 169: 667–680. [DOI] [PubMed] [Google Scholar]
- Knudsen JT, Mori SA. 1996. Floral scents and pollination in neotropical Lecythidaceae. Biotropica 28: 42–60. [Google Scholar]
- Knudsen JT, Eriksson R, Gershenzon J, Ståhl B. 2006. Diversity and distribution of floral scent. Botanical Review 72: 1–120. [Google Scholar]
- Kula AA, Castillo DM, Dudash MR, Fenster CB. 2014. Interactions between a pollinating seed predator and its host plant: the role of environmental context within a population. Ecology and Evolution 4: 2901–2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labouche AM., Bernasconi G. 2010. Male moths provide pollination benefits in the Silene latifolia–Hadena bicruris nursery pollination system. Functional Ecology 24: 534–544. [Google Scholar]
- Light DM, Flath RA, Buttery RG, et al. 1993. Host-plant green-leaf volatiles synergize the synthetic sex pheromones of the corn earworm and codling moth (Lepidoptera). Chemoecology 4: 145–152. [Google Scholar]
- Lindman CAM. 1897. Remarques sur la floraison du genre Silene L. Acta Horti Bergiana 3: 3–28. [Google Scholar]
- Linnaeus C. 1783. Philosophia Botanica. Vienna. [Google Scholar]
- Martinell MC, Dötterl S, Blanché C, Rovira A, Massó S, Bosch M. 2010. Nocturnal pollination of the endemic Silene sennenii (Caryophyllaceae): an endangered mutualism? Plant Ecology 211: 203–218. [Google Scholar]
- May PG. 1988. Determinants of foraging profitability in two nectarivorous butterflies. Ecological Entomology 13: 171–184. [Google Scholar]
- McClung CR. 2006. Plant circadian rhythms. The Plant Cell 18: 792–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meagher RL. 2002. Trapping noctuid moths with synthetic floral volatile lures. Entomologia Experimentalis et Applicata 103: 219–226. [Google Scholar]
- Mendiburu F. 2014. agricolae: Statistical procedures for agricultural research. R package version 1.2-1.
- Menzel R, Shmida A. 1993. The ecology of flower colours and the natural colour vision of insect pollinators: the Israeli flora as a study case. Biological Reviews 68: 81–120. [Google Scholar]
- Milet-Pinheiro P, Ayasse M, Schlindwein C, Dobson HE, Dötterl S. 2012. Host location by visual and olfactory floral cues in an oligolectic bee: innate and learned behavior. Behavioral Ecology 23: 531–538. [Google Scholar]
- Miller TJ, Raguso RA, Kay KM. 2014. Novel adaptation to hawkmoth pollinators in Clarkia reduces efficiency, not attraction of diurnal visitors. Annals of Botany 113: 317–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake T, Yahara T. 1999. Theoretical evaluation of pollen transfer by nocturnal and diurnal pollinators: when should a flower open? Oikos 86: 233–240. [Google Scholar]
- Nepi M, Franchi GG, Padni E. 2001. Pollen hydration status at dispersal: cytophysiological features and strategies. Protoplasma 216: 171–180. [DOI] [PubMed] [Google Scholar]
- Nobel PS. 1977. Water relations of flowering of Agave deserti. Botanical Gazette 138: 1–6. [Google Scholar]
- Ollerton J. 1996. Reconciling ecological processes with phylogenetic patterns: the apparent paradox of plant–pollinator systems. Journal of Ecology 84: 767–769. [Google Scholar]
- Ollerton J, Killick A, Lamborn E, Watts S, Whiston M. 2007. Multiple meanings and modes: on the many ways to be a generalist flower. Taxon 56: 717–728. [Google Scholar]
- Ollerton J, Alarcón R, Waser NM, et al. 2009. A global test of the pollination syndrome hypothesis. Annals of Botany 103: 1471–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollerton J, Rech AR, Waser NM, Mary V., Price MV. 2015. Using the literature to test pollination syndromes – some methodological cautions. Journal of Pollination Ecology 16: 119–125. [Google Scholar]
- Pacini E, Nepi M. 2007. Nectar production and presentation In: Nicolson SW, Nepi M, Pacini E, eds. Nectaries and nectar. Berlin: Springer, 167–214. [Google Scholar]
- Palmer JH, Asprey GF. 1958. Studies in the nyctinastic movement of the leaf pinnae of Samanea saman (Jacq.) Merrill. Planta 51: 770–785. [Google Scholar]
- Pettersson MW. 1991. Pollination by a guild of fluctuating moth populations: option for unspecialization in Silene vulgaris. Journal of Ecology 79: 591–604. [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D, the R Development Core Team. 2013. nlme: linear and nonlinear mixed effects models. R package version 3.1-111.
- Prieto-Benítez S, Dötterl S, Giménez-Benavides L. 2015. Diel variation in flower scent reveals poor consistency of diurnal and nocturnal pollination syndromes in Sileneae. Journal of Chemical Ecology 41: 1095–1104. [DOI] [PubMed] [Google Scholar]
- Proctor M, Yeo P, Lack A. 1996. The natural history of pollination. HarperCollins. [Google Scholar]
- van Putten WF, Elzinga JA, Biere A. 2007. Host fidelity of the pollinator guilds of Silene dioica and Silene latifolia: possible consequences for sympatric host race differentiation of a vectored plant disease. International Journal of Plant Sciences 168: 421–434. [Google Scholar]
- R Core Team. 2014. R: a language and environment for statistical computing R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/.
- Ram HYM, Rao IVR. 1984. Physiology of flower bud growth and opening. Proceedings of the Indian Academy of Sciences 93: 253–274. [Google Scholar]
- Reynolds RJ, Westbrook MJ, Rohde AS, Cridland JM, Fenster CB, Dudash MR. 2009. Pollinator specialization and pollination syndromes of three related North American Silene. Ecology 90: 2077–2087. [DOI] [PubMed] [Google Scholar]
- Reynolds RJ, Kula AA, Fenster CB, Dudash MR. 2012. Variable nursery pollinator importance and its effect on plant reproductive success. Oecologia 168: 439–448. [DOI] [PubMed] [Google Scholar]
- Rhizopoulou S, Ioannidi E, Alexandredes N, Argiropoulos A. 2006. A study on functional and structural traits of the nocturnal flowers of Capparis spinosa L. Journal of Arid Environments 66: 635–647. [Google Scholar]
- Rosas-Guerrero V, Aguilar R, Martén-Rodríguez S, et al. 2014. A quantitative review of pollination syndromes: do floral traits predict effective pollinators? Ecology Letters 17: 388–400. [DOI] [PubMed] [Google Scholar]
- Sanchez A, Shin J, Davis SJ. 2011. Abiotic stress and the plant circadian clock. Plant Signaling and Behavior 6: 223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satter RL, Galston AW. 1981. Mechanisms of control of leaf movements. Annual Review of Plant Physiology 32: 83–110. [Google Scholar]
- Sobrevila C, Arroyo MTK. 1982. Breeding systems in a montane tropical cloud forest in Venezuela. Plant Systematics and Evolution 140: 19–37. [Google Scholar]
- Strauss SY, Whittall JB. 2006. Non-pollinator agents of selection on floral traits In: Harder LD, Barret SCH, eds. Ecology and evolution of flowers. Oxford: Oxford University Press, 120–138. [Google Scholar]
- Talavera S. 1990. Silene L. In: Castroviejo S, Laínz M, López G, et al. , eds. Claves de Flora Ibérica: plantas vasculares de la Península Ibérica e Islas Baleares Madrid: Real Jardín Botánico, CSIC, 313–406 [Google Scholar]
- Tascheri R, Saavedra-Nievas JC, Roa-Ureta R. 2010. Statistical models to standardize catch rates in the multi-species trawl fishery for Patagonian grenadier (Macruronus magellanicus) off Southern Chile. Fisheries Research 105: 200–214. [Google Scholar]
- Teixido AL, Valladares F. 2013. Large and abundant flowers increase indirect costs of corollas: a study of coflowering sympatric Mediterranean species of contrasting flower size. Oecologia 173: 73–81. [DOI] [PubMed] [Google Scholar]
- Teixido AL, Valladares F. 2014. Disproportionate carbon and water maintenance costs of large corollas in hot Mediterranean ecosystems. Perspectives in Plant Ecology, Evolution and Systematics 16: 83–92. [Google Scholar]
- Thompson JN. 1994. The coevolutionary process. Chicago, IL: University of Chicago Press. [Google Scholar]
- Thompson JN. 1999. Specific hypotheses on the geographic mosaic of coevolution. American Naturalist 153: S1–S14. [Google Scholar]
- Tschapka M, von Helversen O. 2007. Phenology, nectar production and visitation behaviour of bats on the flowers of the bromeliad Werauhia gladioliflora in a Costa Rican lowland rain forest. Journal of Tropical Ecology 23: 385–395. [Google Scholar]
- Valiente-Banuet A, Rojas-Martı´nez A, Casas A, del Coro Arizmendi M, Dávila P. 1997. Pollination biology of two winter-blooming giant columnar cacti in the Tehuacán Valley, central Mexico. Journal of Arid Environments 37: 331–341. [Google Scholar]
- Verdonk JC, De Vos CR, Verhoeven HA, Haring MA, van Tunen AJ, Schuurink RC. 2003. Regulation of floral scent production in petunia revealed by targeted metabolomics. Phytochemistry 62: 997–1008. [DOI] [PubMed] [Google Scholar]
- Visser JH, van Straten S, Maarse H. 1979. Isolation and identification of volatiles in the foliage of potato, Solanum tuberosum, a host plant of the Colorado beetle, Leptinotarsa decemlineata. Journal of Chemical Ecology 5: 13–25 [Google Scholar]
- Vogel S. 2006. Floral syndromes: empiricism versus typology. Botanische Jahrbücher für Systematik 127: 5–11. [Google Scholar]
- Waser NM, Chittka L, Price MV, Williams NM, Ollerton J. 1996. Generalization in pollination systems, and why it matters. Ecology 77: 1043–1060. [Google Scholar]
- Waser NM, Ollerton J, eds. 2006. Plant–pollinator interactions: from specialization to generalization. Chicago, IL: University of Chicago Press. [Google Scholar]
- Waser NM, Ollerton J, Erhardt A. 2011. Typology in pollination biology: lessons from an historical critique. Journal of Pollination Ecology 3: 1–7. [Google Scholar]
- Witt T, Jürgens A, Geyer R, Gottsberger G. 1999. Nectar dynamics and sugar composition in flowers of Silene and Saponaria species (Caryophyllaceae). Plant Biology 1: 334–345. [Google Scholar]
- Witt T, Jürgens A, Gottsberger G. 2013. Nectar sugar composition of European Caryophylloideae (Caryophyllaceae) in relation to flower length, pollination biology and phylogeny. Journal of Evolutionary Biology 26: 2244–2259. [DOI] [PubMed] [Google Scholar]
- Wolff D, Braun M, Liede S. 2003. Nocturnal versus diurnal pollination success in Isertia laevis (Rubiaceae): a sphingophilous plant visited by hummingbirds. Plant Biology 5: 71–78. [Google Scholar]
- Young HJ, Gravitz L. 2002. The effects of stigma age on receptivity in Silene alba (Caryophyllaceae). American Journal of Botany 89: 1237–1241. [DOI] [PubMed] [Google Scholar]
- Zapata TR, Arroyo MTK. 1978. Plant reproductive ecology of a secondary deciduous tropical forest in Venezuela. Biotropica 10: 221–230. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.