Table 2.
Activity of C-4 and C-5 substituted pyridazinones 13a–d and 29a–b.
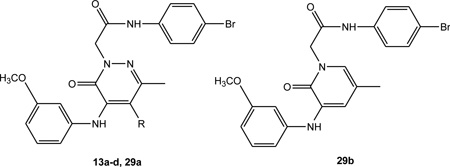
| |||||
|---|---|---|---|---|---|
|
| |||||
| Compd | R | hPMN[a] | FPR1-HL60[a] | FPR2-HL60[a] | FPR3-HL60[a] |
| 13a | CH2CH3 | 0.34 ± 0.11 (115) | 0.18 ± 0.004 (185) | 0.061 ± 0.022 (35) | 0.46 ± 0.014 (35) |
| 13b | CH2CH2CH3 | N.A. | N.A. | N.A. | N.A. |
| 13c | (CH2)3CH3 | 1.40 ± 0.8 (45) | 3.6 ± 1.1 (65) | 4.5 ± 1.3 (30) | N.A. |
| 13d | (CH2)4CH3 | 5.70 ± 1.2 (55) | 1.4 ± 0.34 (95) | 0.19 ± 0.018 (115) | N.A. |
| 29a | H | 0.56 ± 0.12 (85) | 0.24 ± 0.09 (120) | 9.6 ± 2.0 (65) | N.A. |
| 29b | - | 4.31 ± 0.4 (60) | 2.50 ± 0.7 (110) | 8.10 ± 1.7 (75) | N.A. |
Values, expressed as EC50 (µM) and Efficacy (% in brackets) were evaluated in Ca2+ flux assay. EC50 values represent the average mean of three independent experiments and were determined by nonlinear regression analysis of the concentration-response curves (5–6 points) generated using GraphPad Prism 5 with 95% confidential interval (p < 0.05). Efficacy (in brackets) is expressed as % of the response induced by 5 nM fMLF in human polymorphonuclear neutrophils (hPMN) and FPR1-HL60 cells or by 5 nM WKYMVm in FPR2-HL60 and FPR3-HL60 cells.
N.A., no activity (no response was observed during first 2 min after addition of compounds under investigation) considering the limits of efficacy < 20% and EC50 < 50 µM.
