Abstract
The aim of the study was to evaluate the activity of high-polyphenolic black currant (Ribes nigrum L.) and bilberry (Vaccinium myrtillus L.) juices against bacterial strains Asaia lannensis and Asaia bogorensis isolated as spoilage of commercial soft drinks. The composition of fruit juices was evaluated using chromatographic techniques HPLC and LC-MS. The adhesion to glass, polystyrene, and polyethylene terephthalate in two different culture media was evaluated by luminometry and the plate count method. The major anthocyanins in the V. myrtillus were petunidin-3-glucoside, malvidin-3-glucoside, cyanidin-3-glucoside, and delphinidin-3-glucoside, while in R. nigrum delphinidin-3-rutinoside and cyanidin-3-rutinoside were detected. The LC-MS analysis showed presence of anthocyanins (delphinidin, cyanidin, petunidin, and malvidin derivatives), phenolic acids (chlorogenic and neochlorogenic acids), flavonols (quercetin-3-glucoside, quercetin-3-rutinoside), and flavanols (procyanidin B2 and procyanidin type A2). Additionally, in the bilberry juice A type procyanidin trimer was detected. The adhesion of Asaia spp. cells depended on the type of medium, carbon sources, and the type of abiotic surfaces. We noted that the adhesion was significantly stronger in minimal medium containing sucrose. The addition of bilberry and black currant juices notably reduced bacterial growth as well as cell adhesion to polyethylene terephthalate surfaces.
1. Introduction
Nowadays, consumers are increasingly interested in their health and expect the foods, besides possessing the sensory attractiveness, to have health-promoting effects. Numerous studies indicate that a diet rich in berries and their preserves positively affects human health. Regular consumption of fruits may delay ageing processes and reduce the risk of various illnesses, such as cancer, cardiovascular and lung diseases, rheumatoid arthritis, Alzheimer's dementia, or Parkinsonism [1–3]. Fruit berries were identified as sources of phenolic compounds like gallic and ellagic acid with potential cancer chemopreventive activity. The different bioactive phenolic compounds, including flavonoids (flavonols and flavanols), tannins (proanthocyanidins, ellagitannins, and gallotannins), stilbenoids, and phenolic acids, have received considerable interest in bearing possible relations to human health [1]. Besides health-promoting properties, polyphenols may also act as antimicrobials and antiadhesive agents in wide range of pathogens [4]. It was documented that berry extracts or juices showed strong activity against Gram negative bacteria [5, 6]. In the past decade, cranberry extracts were attracting ever-growing attention of microbiologists. It was noted that cranberry polyphenol fraction inhibits growth and adhesion of urinary tract pathogens (Escherichia coli, Proteus vulgaris), Helicobacter pylori, and bacterial etiological factors of oral diseases (Streptococcus spp., Propionibacterium spp., and Fusobacterium spp.) [7–12].
Lately, numerous reports detailed various spoilage microorganisms in soft drinks, for example, acetic acid bacteria belonging to the genus Asaia [13–15]. The growth of these microorganisms causes significant changes in both microbiological and organoleptic qualities. Asaia spp. cells are able to grow in soft drinks supplemented with different preservatives (benzoate, sorbate, and dimethyl dicarbonate) [15]. What is more, these bacteria show strong adhesive abilities on food-contact technical materials. The biofilm formed by Asaia species on solid surfaces of a production line can be a source of secondary contamination of final products [16].
The initial, key step leading to biofilm formation is bacterial adhesion to the surface. This is the complex process, influenced by various physical and chemical properties of microbial cells, media, and abiotic surfaces. Among these factors, modification of media could be changed in order to prevent biofouling in soft drinks technology. New antimicrobial strategy is the use of berry juices to inhibit or reduce bacterial adhesion. The application of native and low-priced fruits with additional potential as health-promoting agents is especially interesting. Therefore, the aim of our study was to investigate antibacterial and antiadhesion activities of juices from bilberries and black currants against Asaia spp. cells.
2. Materials and Methods
2.1. Plant Material
The black currant (R. nigrum L.) and bilberry (V. myrtillus L.) fruits were freshly harvested from the local orchard and forests around Lodz (central Poland). The fruits were washed with sterile water, lightly air-dried, and frozen at −20°C for one month. The fresh juice was squeezed out from defrosted fruits using extractor MES3000 (Bosch, Poland). Cloudy juice was clarified using Whatman qualitative paper-filter and then by 0.45 μm filtration (Filter-Bio). Immediately after preparation, the clear juice was added to the culture media to the final concentration of 10% (v/v).
2.2. Bacterial Strains and Culture Media
The study used the six bacterial strains: Asaia bogorensis ISD1 (GenBank KP234014), A. bogorensis ISD2 (GenBank KP234015), A. bogorensis FFMW (GenBank KC756841), A. lannensis IFMW (GenBank KP234011), A. lannensis IFCW (GenBank KP234012), and A. lannensis FMW1 (GenBank HQ917850) isolated from spoiled flavored mineral water and isotonic drinks. These strains were identified using morphological, physiological, and genetic methods described by Kregiel and coworkers [13, 17]. The obtained nucleotide sequences of 16S rRNA were deposited in GenBank (National Centre of Biotechnology Information) and the bacterial strains were deposited in the Pure Culture Collection of Industrial Microorganisms LOCK 105 at the Institute of Fermentation Technology and Microbiology, Technical University of Lodz (Poland).
The adhesion was investigated in liquid culture media: the rich GC medium (M1) (0.3% (w/v) peptone, 0.3% (w/v) yeast extract) and the minimal medium (M2) (0.3% (NH4)2PO4 (w/v), 0.3% KH2PO4 (w/v), 0.3% MgSO4 × 7H2O (w/v), and 0.05% (w/v) yeast extract). In both media, carbohydrates, glucose, fructose, and sucrose (2% w/v), were used as a carbon source. The sterile media (20 cm3) were poured aseptically into 25 cm3 Erlenmeyer flasks covered with a textile cloth in order to ensure aerobic conditions. Sterile carriers were placed vertically in a liquid culture medium in such a way that half of the carrier was immersed in the medium, and the other part was above the liquid.
2.3. Carriers
The bacterial adhesion was carried out to the polystyrene (PS) (Coveris Rigid Poland, Skierniewice) and polyethylene terephthalate (PET) (Coveris Rigid Poland, Skierniewice) slides measuring 76 × 26 mm. These materials are certified by Polish National Institute of Public Health and approved for contact with food. The white glass slides (G) (Knittel Glass, Germany) were used as the reference material. Carriers were sterilized in two-step process. First, the carriers were kept in the 70% ethanol solution for 3 hours. Subsequently, they were placed in a laminar chamber and subjected to UV irradiation for 2 hours.
2.4. Adhesion Analysis
Studies on the Asaia spp. attachment and biofilm formation were carried out in two stages. The first stage involved the selection of a culture medium, a carbon source, and an abiotic material where bacteria demonstrated the strongest adhesion abilities. In the second stage, we checked the effect of fruit juices on the growth and adhesion abilities of Asaia spp. For this purpose, the culture medium containing selected carbon source, with proper carrier, was supplemented with 10% (v/v) of black currant or bilberry juice.
At the beginning of the experiments culture media were inoculated with standardized bacterial suspensions, to obtain cell concentration 105 ÷ 106 CFU/cm3. The adhesion ability of the bacterial strains was evaluated according to the method described by Kregiel (2013) [16]. For luminometric tests, the carriers were removed from the culture media, washed with sterile distilled water, and swabbed with pens for ATP sampling (Merck). Measurements were made in relative light units (RLU) using a HY-LiTE® 2 luminometer (Merck). The plate count method was used in order to determine the number of cells attached to the carrier and planktonic cells in the culture medium. The carrier plate was removed from the culture medium, rinsed with sterile distilled water, and swabbed using sterile swabs for surface testing. The bacterial suspensions were vortexed with 0.1% (v/v) Tween 80 and transferred onto GC agar medium supplemented with 0.7% CaCO3 (w/v), and after incubation at 25°C for 92 h the colonies were counted. The number of colony forming units (CFU) per cm3 (of liquid media) or per cm2 (of carriers) was calculated. On the basis of the results, the relative adhesion coefficient A (%) was calculated using formula A (%) = (N a/N p) × 100%, where N a is the number of attached cells to a carrier and N p is the number of planktonic cells in the culture medium.
2.5. Chemical Constituent's Analysis
The organic acids and carbohydrates profiles of the tested fruit juices were determined using high performance liquid chromatography (HPLC), according to the method described by Gutarowska and Czyżowska (2009) [18]. In addition, the polyphenolic compounds were also characterized using HPLC-DAD method with a diode array detector (Finnigan Surveyor-PDA Plus detector) and a ChromQuest 5.0 chromatography software (Thermo Fisher Scientific Inc., Waltham, MA, USA) as well as using liquid chromatography mass spectrometry (LC-MS; LTQ Velos MS, Thermo Fisher Scientific) following the method described by Antolak et al. (2015) [19].
2.6. Statistics
Means were calculated from the data obtained from three independent experiments, and the standard deviations (SD) were calculated. The mean values of the adhesion results were compared using one-way repeated measures analysis of variance with Tukey test (ANOVA; OriginPro 8.1, OriginLab Corp., Northampton, MA). Statistical significance was set at the conventional level of 5% (p < 0.05).
3. Results and Discussion
3.1. Bacterial Adhesion
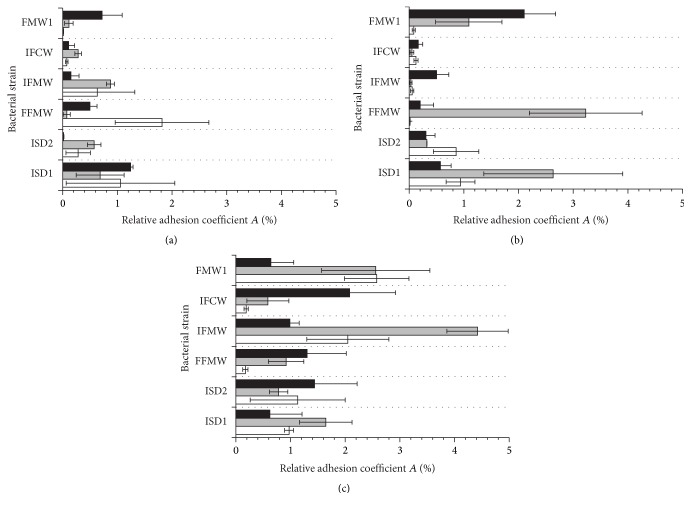
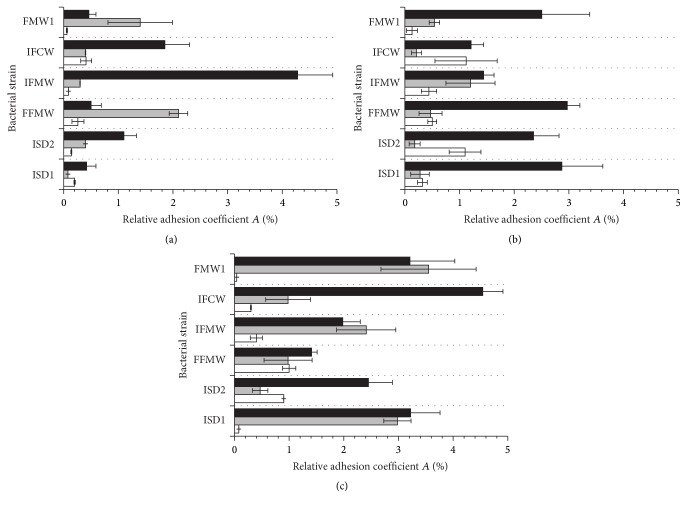
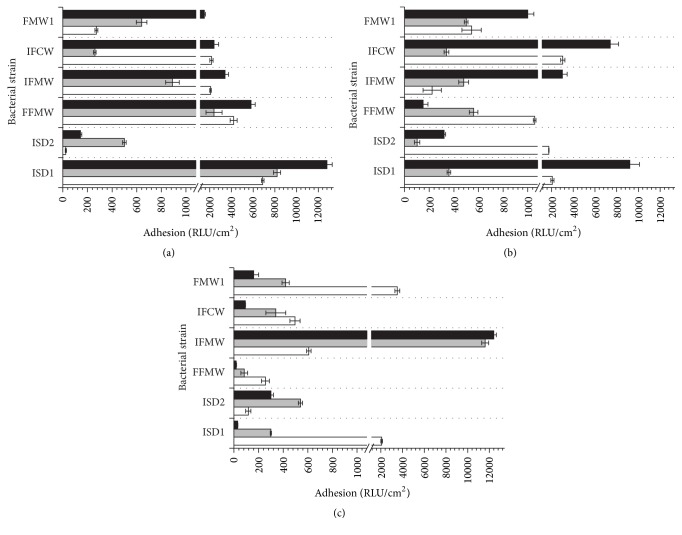
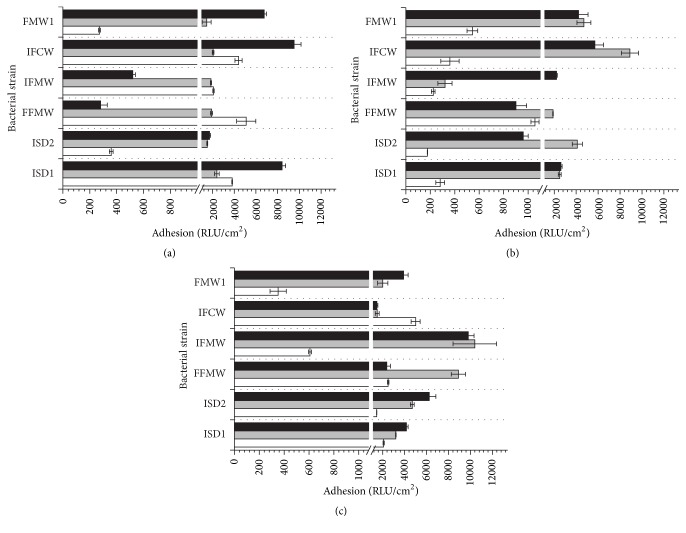
To determine the level of bacterial adhesion, two main analytical methods, namely, plate count and luminometry, were used. The evaluation of Asaia spp. adhesion to glass, polystyrene, and polyethylene terephthalate surfaces was carried out in rich M1 and minimal M2 medium. The influence of the carbon source for bacterial adhesion was tested in culture media supplemented with glucose, fructose, or sucrose as an only carbon source. The results of adhesion studies, expressed as relative adhesion coefficient A (%) for medium M1 and medium M2, are presented in Figures 1 and 2, respectively. The biofilm formation of Asaia strains significantly increased in culture media supplemented with sucrose (p < 0.05) in comparison to media containing glucose or fructose. It was noted that the minimal M2 medium was a more favorable environment for the Asaia spp. adhesion and biofilm formation compared to the rich M1 medium. The results for adhesion in M2 medium, with reference to those obtained for the adhesion in M1 medium, were significantly higher (p < 0.05). The average value of A (%) for cells adhesion in M2 medium with sucrose was 1.72 ± 0.26%, while for the same medium but with fructose and glucose the results were slight lower and equaled 1.10 ± 0.23% (p = 0.00001) and 0.80 ± 0.19% (p = 0.00004), respectively. The highest value of A (%) was noted for A. lannensis IFCW strain on PET surface, which was 4.54 ± 0.37%. Figures 3 and 4 present the luminometry results (RLU/cm2) obtained for bacterial adhesion in M1 and M2 media, respectively. The obtained results confirmed that the more favorable environment for biofilm formation is the minimal medium M2 with sucrose. Average value of the RLU for rich M1 medium with sucrose (1784 ± 257 RLU/cm2) was statistically lower (p = 0.001) in comparison to minimal medium M2 with the same carbohydrate (3923 ± 447 RLU/cm2).
Figure 1.
The relative adhesion coefficient A (%) for A. bogorensis (ISD1, ISD2, and FFMW) and A. lannensis (IFMW, IFCW, and FMW1) strains in M1 medium with glucose (a), fructose (b), and sucrose (c) to PET (black bars), PS (grey bars), and G (white bars).
Figure 2.
The relative adhesion coefficient A (%) for A. bogorensis (ISD1, ISD2, and FFMW) and A. lannensis (IFMW, IFCW, and FMW1) strains in M2 medium with glucose (a), fructose (b), and sucrose (c) to PET (black bars), PS (grey bars), and G (white bars).
Figure 3.
The adhesion (RLU/cm2) of A. bogorensis (ISD1, ISD2, and FFMW) and A. lannensis (IFMW, IFCW, and FMW1) strains in M1 medium with glucose (a), fructose (b), and sucrose (c) to PET (black bars), PS (grey bars), and G (white bars).
Figure 4.
The adhesion (RLU/cm2) of A. bogorensis (ISD1, ISD2, and FFMW) and A. lannensis (IFMW, IFCW, and FMW1) strains in M2 medium with glucose (a), fructose (b), and sucrose (c) to PET (black bars), PS (grey bars), and G (white bars).
Additionally, to assess the differences between the adhesion abilities of all bacterial strains to all tested carriers in all culture media containing different carbon sources, the mean values and standard deviations calculated from obtained results of A (%) (Table 1) and the RLU/cm2 (Table 2) were calculated. It was noted that the adhesion and biofilm formation processes were strain-dependent. A. lannensis strains showed slightly stronger adhesion in culture media containing sucrose. The mean A (%) values for A. lannensis strains adhesion to PET surface in culture media with sucrose were 1.23 ± 0.61% (M1) and 3.24 ± 1.05% (M2) while for the A. bogorensis strains 1.12 ± 0.36% (p = 0.05) and 2.36 ± 0.74% (p = 0.02) were noted, respectively.
Table 1.
Adhesion of the Asaia spp. strains reported as relative coefficient A (%) in M1 and M2 media with carbohydrates as a carbon source. The mean values of the adhesion results were compared using one-way repeated measures ANOVA with Tukey test. Two results of p values were obtained: p 1 – p value obtained by the comparison of the A (%) results within a species to the results for M1 with glucose and glass; p 2 – p value obtained by the comparison of the A (%) between Asaia bogorensis and Asaia lannensis. Statistical significance was set at the conventional level of 5% (p < 0.05).
| Species | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A. bogorensis | A. lannensis | |||||||||||
| Carbon source | ||||||||||||
| Glucose | Fructose | Sucrose | Glucose | Fructose | Sucrose | |||||||
| Medium | ||||||||||||
| M1 | M2 | M1 | M2 | M1 | M2 | M1 | M2 | M1 | M2 | M1 | M2 | |
| Surface | ||||||||||||
|
| ||||||||||||
| G | 1.05 ± 0.63 p 2 = 0.11 |
0.20 ± 0.05 p 1 = 0.11 p 2 = 0.04 |
0.60 ± 0.42 p 1 = 0.02 p 2 = 0.14 |
0.64 ± 0.33 p 1 = 0.03 p 2 = 0.14 |
0.76 ± 0.42 p 1 = 0.01 p 2 = 0.04 |
0.66 ± 0.41 p 1 = 0.09 p 2 = 0.05 |
0.24 ± 0.28 p 2 = 0.11 |
0.19 ± 0.16 p 1 = 0.15 p 2 = 0.04 |
0.09 ± 0.03 p 1 = 0.21 p 2 = 0.14 |
0.56 ± 0.41 p 1 = 0.14 p 2 = 0.14 |
1.60 ± 1.03 p 1 = 0.15 p 2 = 0.04 |
0.25 ± 0.16 p 1 = 0.23 p 2 = 0.05 |
|
| ||||||||||||
| PS | 0.44 ± 0.27 p 1 = 0.04 p 2 = 0.11 |
0.86 ± 0.89 p 1 = 0.19 p 2 = 0.25 |
2.06 ± 1.25 p 1 = 0.14 p 2 = 0.15 |
0.31 ± 0.12 p 1 = 0.12 p 2 = 0.09 |
1.11 ± 0.38 p 1 = 0.05 p 2 = 0.12 |
1.48 ± 1.08 p 1 = 0.11 p 2 = 0.09 |
0.42 ± 0.33 p 1 = 0.26 p 2 = 0.11 |
0.70 ± 0.50 p 1 = 0.07 p 2 = 0.25 |
0.39 ± 0.50 p 1 = 0.16 p 2 = 0.15 |
0.65 ± 0.41 p 1 = 0.20 p 2 = 0.09 |
2.52 ± 1.57 p 1 = 0.16 p 2 = 0.12 |
2.31 ± 1.05 p 1 = 0.08 p 2 = 0.09 |
|
| ||||||||||||
| PET | 0.59 ± 0.51 p 1 = 0.13 p 2 = 0.15 |
0.67 ± 0.30 p 1 = 0.02 p 2 = 0.11 |
0.36 ± 0.16 p 1 = 0.08 p 2 = 0.14 |
2.73 ± 0.27 p 1 = 0.02 p 2 = 0.02 |
1.12 ± 0.36 p 1 = 0.04 p 2 = 0.05 |
2.36 ± 0.74 p 1 = 0.01 p 2 = 0.02 |
0.33 ± 0.28 p 1 = 0.09 p 2 = 0.15 |
2.20 ± 1.58 p 1 = 0.20 p 2 = 0.11 |
0.92 ± 0.84 p 1 = 0.17 p 2 = 0.14 |
1.72 ± 0.56 p 1 = 0.03 p 2 = 0.02 |
1.23 ± 0.61 p 1 = 0.08 p 2 = 0.05 |
3.24 ± 1.05 p 1 = 0.03 p 2 = 0.02 |
Table 2.
Adhesion of the Asaia spp. strains, reported in RLU/cm2, in M1 and M2 media with carbohydrates as a carbon source. The mean values of the adhesion results were compared using one-way repeated measures ANOVA with Tukey test. Two results of p values were obtained: p 1 – p value obtained by the comparison of the RLU/cm2 results within a species to the results for M1 with glucose and glass; p 2 – p value obtained by the comparison of the RLU/cm2 between Asaia bogorensis and Asaia lannensis. Statistical significance was set at the conventional level of 5% (p < 0.05).
| Species | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A. bogorensis | A. lannensis | |||||||||||
| Carbon source | ||||||||||||
| Glucose | Fructose | Sucrose | Glucose | Fructose | Sucrose | |||||||
| Medium | ||||||||||||
| M1 | M2 | M1 | M2 | M1 | M2 | M1 | M2 | M1 | M2 | M1 | M2 | |
| Surface | ||||||||||||
|
| ||||||||||||
| G | 3701 ± 2821 p 2 = 0.12 |
3086 ± 2000 p 1 = 0.17 p 2 = 0.01 |
1632 ± 428 p 1 = 0.12 p 2 = 0.09 |
504 ± 393 p 1 = 0.18 p 2 = 0.13 |
817 ± 894 p 1 = 0.22 p 2 = 0.13 |
2010 ± 432 p 1 = 0.12 p 2 = 0.08 |
1504 ± 871 p 2 = 0.12 |
2251 ± 1690 p 1 = 0.16 p 2 = 0.01 |
1269 ± 1259 p 1 = 0.16 p 2 = 0.09 |
376 ± 131 p 1 = 0.07 p 2 = 0.13 |
1541 ± 1400 p 1 = 0.01 p 2 = 0.13 |
1987 ± 2133 p 1 = 0.21 p 2 = 0.08 |
|
| ||||||||||||
| PS | 3700 ± 3375 p 1 = 0.22 p 2 = 0.22 |
1937 ± 364 p 1 = 0.13 p 2 = 0.01 |
340 ± 187 p 1 = 0.19 p 2 = 0.05 |
2830 ± 932 p 1 = 0.05 p 2 = 0.13 |
308 ± 186 p 1 = 0.17 p 2 = 0.36 |
5600 ± 2412 p 1 = 0.06 p 2 = 0.04 |
597 ± 259 p 1 = 0.07 p 2 = 0.22 |
1783 ± 249 p 1 = 0.05 p 2 = 0.01 |
440 ± 71 p 1 = 0.08 p 2 = 0.05 |
4640 ± 3503 p 1 = 0.13 p 2 = 0.13 |
4120 ± 5289 p 1 = 0.30 p 2 = 0.36 |
4633 ± 4082 p 1 = 0.19 p 2 = 0.04 |
|
| ||||||||||||
| PET | 6247 ± 5177 p 1 = 0.22 p 2 = 0.16 |
3463 ± 3539 p 1 = 0.23 p 2 = 0.02 |
3223 ± 4227 p 1 = 0.28 p 2 = 0.16 |
1487 ± 788 p 1 = 0.17 p 2 = 0.01 |
817 ± 130 p 1 = 0.18 p 2 = 0.40 |
4252 ± 1591 p 1 = 0.04 p 2 = 0.05 |
2433 ± 776 p 1 = 0.07 p 2 = 0.16 |
5590 ± 3757 p 1 = 0.11 p 2 = 0.02 |
3800 ± 2673 p 1 = 0.15 p 2 = 0.16 |
4033 ± 1434 p 1 = 0.04 p 2 = 0.01 |
4217 ± 5786 p 1 = 0.32 p 2 = 0.40 |
5053 ± 3501 p 1 = 0.13 p 2 = 0.05 |
A. lannensis and A. bogorensis were characterized by stronger adhesion properties to plastic materials in comparison to the glass surface. The average values of the relative adhesion coefficient obtained for the carriers in minimal medium M2 with sucrose were 0.45 ± 0.05% (G), 1.90 ± 0.23% (PS), and 2.80 ± 0.21% (PET), while for rich M1 medium 1.18 ± 0.71%, 1.82 ± 1.01%, and 1.17 ± 0.23% were noted, respectively. Performed ANOVA test showed that the results are statistically different. Obtained p values, in comparison to glass, for the M1 medium were 0.02 (PS) and 0.01 (PET), while the results for M2 medium were less than 0.01 for both PS and PET. The results of RLU measurement also showed that slightly better surface for biofilm formation in M2 medium with sucrose is PET.
The similar results for Asaia spp. adhesion were obtained by Kregiel (2013) and Kregiel et al. (2014), where, after incubation, the adhesion to plastic materials was several times higher in comparison to the glass surface [16, 17].
Of course, there are different techniques that can be used in the analysis of the microbial adhesion to abiotic surfaces, but neither method is perfect. The plate count technique in particular allows determining culturable microorganisms, while luminometric methods enable estimating total biological material on the abiotic surfaces. This approach is based on bacterial ATP quantification and can be used to evaluate not only the total number of adhering cells, but all biomass: bacteria that are able and unable to grow, extracellular polymeric substances, or adhered organic material from culture media. Thus, comparing the results of the relative coefficient A (%) and RLU/cm2, the values obtained by these two methods showed differences.
The type of material, its roughness, and hydrophobicity significantly affect bacterial attachment and biofilm development. The plastic materials used in our study were characterized by low surface energy (PET 44 mN/m at 20°C, PS 40 mN/m at 20°C) in comparison to hydrophilic glass surface (70 mN/m at 20°C) [17, 20]. What is more, studies confirmed that bacterial adhesion is influenced by many physiochemical properties of the environment, the availability and type of carbon source, and type of surface and microorganism abilities [21]. These parameters also determine the cell adhesion in industrial conditions. For example, Møretrø and Langsrud (2004) reported that food-processing environmental factors, including sugars and nutrients, had significant impacts on Listeria monocytogenes adhesion and biofilm formation [22]. Therefore, for the next stage of research, involving effect of berries juices on the growth and adhesion of Asaia spp., we choose rich M1 medium with glucose and minimal M2 medium with sucrose, respectively.
3.2. Chromatographic Analysis of Juices
The carbohydrate profiles of the fruit extracts indicated that the main sugars were glucose and fructose. In the bilberry juice, fructose concentration was 1.94 g/100 mL, while glucose equaled 0.76 g/100 mL. Respectively, for the black currant juice, the values were 0.60 g/100 mL and 0.54 g/100 mL. According to the literature, in the majority of native fruit juices, the content of saccharides is limited only to glucose, fructose, and sucrose. The variability of determined saccharide contents in fruit juices from berries stemmed from differences in variety, stage of ripeness, and climatic conditions [23].
The polyphenolic profiles in fruit juices were determined using HPLC method and the results are presented in Figures 5 and 6. We noted good separation of thirteen anthocyanins in the bilberry juice while for the black currant juice we detected six defined compounds. In the bilberry juice, delphinidin (Dp), cyanidin (Cy), petunidin (Pet), peonidin (Pn), and malvidin (Mal) with galactoside (Gal), glucoside (Glu), and arabinoside (Ara) forms were detected. The results obtained for black currant juice indicate that the material is a source of delphinidin-3-glucoside, delphinidin-3-rutinoside, cyanidin-3-glucoside, and cyanidin-3-rutinoside as well as petunidin-3-glucoside and petunidin-3-rutinoside. The individual anthocyanin contents were determined according to the linear calibration curve (correlation coefficient = 0.989) and expressed as μg of cyanidin-3-glucoside per one mL. The highest concentration of these compounds in the Vaccinium myrtillus juice was noted for petunidin-3-glucoside (2.48 μg/mL) and malvidin-3-glucoside (2.41 μg/mL), cyanidin-3-glucoside (1.83 μg/mL), and delphinidin-3-glucoside (1.78 μg/mL). The major anthocyanins in the Ribes nigrum juice were delphinidin-3-rutinoside (2.04 μg/mL) and cyanidin-3-rutinoside (1.99 μg/mL). The presence of anthocyanins was also confirmed by LC-MS (Table 3). Twenty-two compounds were detected: seven common for both juices, twelve designated only for bilberry, and three for black currant juice. Besides anthocyanins (delphinidin, cyanidin, petunidin, and malvidin derivatives), phenolic acids (chlorogenic and neochlorogenic) as well as flavonols (quercetin-3-glucoside, quercetin-3-rutinoside) and flavanols (procyanidin B2 and procyanidin type A2) were detected. Numerous studies have reported the composition of phenolic acids, anthocyanins, and flavonols in Ribes nigrum [24–26] and Vaccinium myrtillus fruits [27–30]. The bilberry fruits are a rich source of delphinidin, cyanidin, petunidin, peonidin, malvidin, and their derivatives. The anthocyanin concentration of bilberry juices ranged from 1610 to 5963 mg/L, with the mean of 3087 mg/L [30] while in the case of R. nigrum the average content of anthocyanin amounts to 3500 mg/L [31]. In relation to these data, black currant juice used in our study was characterized by much lower content of anthocyanins than bilberry juice, both qualitatively and quantitatively. The variations in anthocyanin profiles may be determined by genotype features of the plants and climatic conditions [28]. Despite the significant differences in the content of polyphenol compounds, juices of black currants and blueberries are rich sources of bioactive compounds that can be used as a remedy in many illnesses. It is well known that these compounds have beneficial effects in preventing cardiovascular and neurological diseases [32, 33] and possess anticancer [34, 35], anti-inflammatory [36, 37], neuroprotective [38], and antidiabetic [39] activities. The antibacterial activities of various fruit extracts on common potential pathogens including antibiotic-resistant strains were also documented [40]. Research suggests that cranberry (Vaccinium macrocarpon) juice, in particular, helps in maintaining the health of the urinary tract [41]. The profile of cranberry juice, being rich in A type proanthocyanidins (PACs) in contrast to the B-type PACs, presents in most other fruits [42]. PACs are colorless oligomers and polymers of flavan-3-ols that show especial antiaggregation abilities [43]. The antibacterial activity of cranberry A type proanthocyanidin was demonstrated in vitro on uropathogenic P-fimbriated Escherichia coli [44] and other pathogenic bacteria [7, 9]. What is interesting is that our results of LC-MS showed that bilberry juice is a source of proanthocyanidins type A and procyanidin type 2. Despite the limited literature concerning the data demonstrating the presence of type A proanthocyanidins in cranberry, some research suggests that they may also be present in wild berries. Schmidt et al. (2004) suggest that high molecular weight oligomeric proanthocyanidins from wild Vaccinium angustifolium exhibit strong antiproliferation activity against human prostate and mouse liver cancer cell lines [45]. Characterization of proanthocyanidins in wild blackberries was also carried out in the work of Cuevas-Rodríguez et al. (2010) [46]. Generally, the highest contents of all types of proanthocyanidins were determined in blackthorns, chokeberries, saskatoon berries, blueberries, cranberries, and lingonberries [46–52]. Moreover, it was shown that the proanthocyanidins can also be present in the bilberry fruits, chemical composition of which may be similar to that of cranberry fruit [53].
Figure 5.
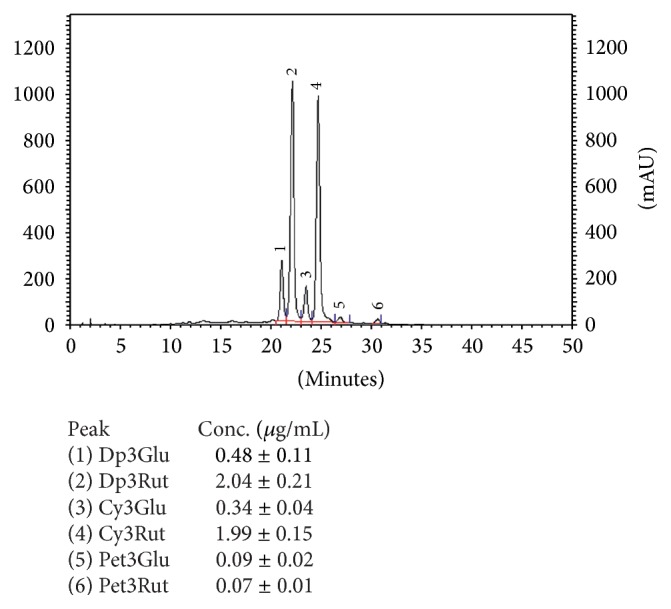
Anthocyanins profile in the Ribes nigrum juice.
Figure 6.
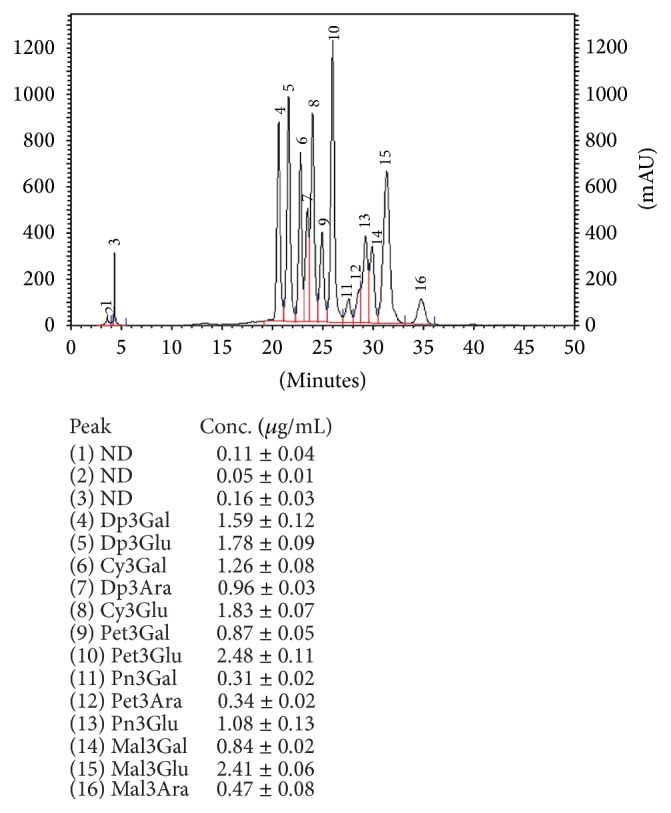
Anthocyanins profile in the Vaccinium myrtillus juice.
Table 3.
Bioactive compounds in bilberry and black currant juices.
| RT (min) | λ max (nm) | [M − H]− | Fragment ions | Compound | V. myrtillus | R. nigrum |
|---|---|---|---|---|---|---|
| 8.48 | 244, 323 | 353 | 191, 179 | Neochlorogenic acid | + | + |
| 9.06 | 244, 330 | 355 | 191 | Chlorogenic acid | − | + |
| 9.62 | 223, 280 | 463 | 301 | Delphinidin-3-galactoside | + | − |
| 9.73 | 246, 330 | 355 | 179, 163 | Caffeoyl hexose | + | + |
| 11.01 | 522 | 341 | 179 | Dicaffeic acid | + | − |
| 11.22 | 278, 521 | 463 | 301 | Delphinidin-3-glucoside | + | + |
| 11.65 | 224, 522 | 609 | 301, 406 | Delphinidin-3-rutinoside | − | + |
| 13.47 | 280, 520 | 447 | 285 | Cyanidin-3-glucoside | + | + |
| 15.60 | 280, 521 | 477 | 315 | Petunidin-3-galactoside | + | + |
| 15.63 | 236, 279 | 577 | 407 | Procyanidin B2 | + | − |
| 15.93 | 236, 280 | 575 | 377, 395, 449 | Procyanidin A2 | + | − |
| 16.04 | 272, 520 | 477 | 315 | Petunidin-3-glucoside | + | − |
| 20.78 | 260, 352 | 479 | 317 | Myricetin-3-galactoside | − | + |
| 20.84 | 254, 354 | 461 | 301 | Quercetin-3-glucoside | + | + |
| 21.20 | 276, 527 | 491 | 329 | Malvidin-3-galactoside | + | + |
| 21.47 | 233, 279 | 866 | 577, 451 | B-type procyanidin trimer | + | − |
| 25.34 | 233, 280 | 863 | 573, 411 | A type procyanidin trimer | + | − |
| 26.45 | 230, 278 | 1152 | 861, 577 | A type procyanidin tetramer | + | − |
| 28.59 | 261, 352 | 479 | 317 | Myricetin-3-glucoside | + | − |
| 28.68 | 233, 279 | 489 | 285 | Cyanidin-6-acetyl-3-glucoside | + | − |
| 30.34 | 281, 521 | 505 | 301 | Delphinidin-6-acetyl-3-glucoside | + | − |
| 33.71 | 258, 354 | 609 | 301 | Quercetin-3-rutinoside | + | − |
3.3. Growth
Due to the higher A (%) results and quite high RLU values, for the next stage of this study, based on the effect of fruit juices on the growth and adhesion of Asaia spp., we chose M1 medium with glucose for growth analysis and M2 medium with sucrose with PET carriers for adhesion investigation.
The growth in M1 medium without fruit juices varied depending on the strain with mean value of 7.02 ± 2.41 × 109 CFU/cm3 (Figure 7). After 14-day incubation, the best growth was noted for A. bogorensis ISD2 (1.08 ± 0.23 × 1010 CFU/cm3) and A. bogorensis ISD1 (9.97 ± 1.45 × 109 CFU/cm3) while the lowest number of the bacteria was detected for A. lannensis FMW1 (2.67 ± 1.70 × 109 CFU/cm3). The addition of R. nigrum and V. myrtillus juices caused a slight reduction in the number of viable bacterial cells. The average count in M1 medium with 10% (v/v) bilberry juice and black currant juice was 2.60 ± 1.35 × 109 CFU/cm3 and 4.37 ± 2.85 × 109 CFU/cm3, respectively. The obtained results suggested that A. bogorensis showed higher sensitivity to fruit juices than A. lannensis strains.
Figure 7.
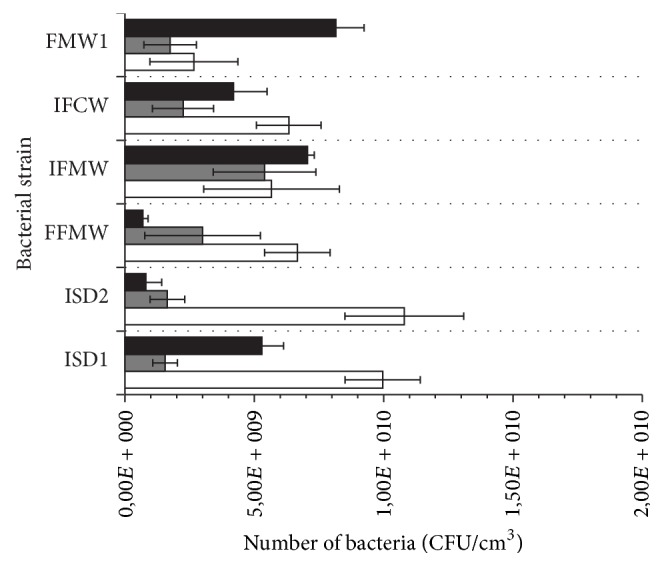
Growth of the Asaia spp. strains in M1 medium with glucose (white bars), supplemented by bilberry (grey bars) and black currant (black bars) juices.
According to the literature, polyphenols from various fruit demonstrate antibacterial activities, especially against pathogenic strains: P. aeruginosa, Staph. aureus, E. coli, L. monocytogenes, and Salmonella spp. Polyphenols are able to suppress a number of microbial virulence factors, such as reduction of host ligands adhesion, inhibition of biofilm formation, and neutralization of bacterial toxins, and show synergism with antibiotics [54]. The activity of phenolic compounds includes interaction with microbial enzymes that are responsible for the cell growth or have direct influence on microbial metabolism by inhibition of oxidative phosphorylation [55]. In addition, the cells of Gram negative bacteria are surrounded by an outer membrane, which acts as barrier protecting against many external agents [56]. The permeability of this membrane is regulated by hydrophilic channels which generally exclude the entry of hydrophobic substances to the bacterial cell. However, some agents, including essential oils and terpenoids and other phenolic compounds, affect membrane barriers, which stimulate the penetration of bioactive agents in bacterial cells [57]. It was found that berries extracts clearly caused higher permeability of Salmonella spp. membranes, cell penetration, and reaction with cellular proteins [58]. According to Nohynek et al. (2006), the activity of polyphenolic compounds from berry fruits may be the result of multiple mechanisms and synergies due to the presence of various bioactive compounds [56]. In Puupponen-Pimiä et al. (2001) study, extracts from blueberry and black currant fruits were checked against pathogenic Gram negative and Gram positive bacteria [5]. It was shown that anthocyanins (pelargonidin, cyanidin) as well flavonols (myricetin) showed inhibitory effect against Gram negative cells of E. coli and Salmonella spp. Phenolic extracts containing tannins and their derivatives showed strong antibacterial effect against Staph. aureus, H. pylori, C. perfringens, B. cereus, Klebsiella spp., and Proteus spp. [56, 58]. However, the knowledge about the effect of fruit phenolics on food spoilage bacteria is still limited.
3.4. Biofouling
It is well known that luminometric measurements in an environment of fruit juices that are rich in polyphenols may carry a margin of error. Luminometry is based on the reaction of enzymatic oxidation of luciferin to oxyluciferin and the presence of antioxidants can influence the final results. It has been documented that polyphenols present in green tea can inhibit the enzymatic activities [59]. Therefore, in the light of that fact, we used two different methods to assess the adhesion of cells to PET surface: luminometry and plate count technique.
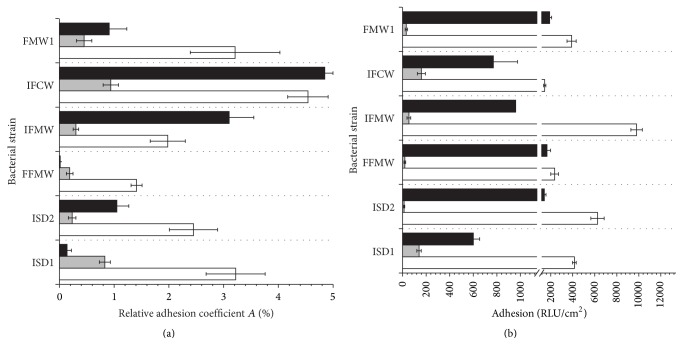
The effect of the bilberry and black currant juices on the adhesion properties of Asaia spp. was performed during cultivation in M2 medium with PET carriers. Results, expressed as adhesion relative coefficient A (%) and RLU/cm2, were presented in Figures 8(a) and 8(b), respectively. The coefficient A (%) calculated for the sixth day of incubation with 10% (v/v) juice showed significant decrease in the adhesion and biofilm formation (Figure 8(a)). This parameter for cell adhesion with bilberry juice ranged from 0.19 ± 0.11% to 0.94 ± 0.59%, while for black currant juice the values were 0.01 ± 0.009% to 4.85 ± 0.41%. The results were 4 ÷ 11 times lower in comparison to the control sample without V. myrtillus juice. Luminometric results (RLU/cm2) also confirmed significant reduction of adhesion (Figure 8(b)). There were statistically significant differences between the control samples and cultures with fruit juices (p < 0.05). Additionally, the differences were noted for antiadhesive activities of tested juices. The values ranged from 1460 ± 102 RLU/cm2 to 9800 ± 520 RLU/cm2 (Av = 4252 ± 2748 RLU/cm2) for the control sample and for adhesion in the presence of V. myrtillus and R. nigrum from 14 ± 5 RLU/cm2 to 160 ± 34 RLU/cm2 (Av = 70 ± 58 RLU/cm2) and from 600 ± 54 RLU/cm2 to 1900 ± 187 RLU/cm2, respectively (Av = 1218 ± 474 RLU/cm2). Thus, bilberry juice inhibited biofouling of all tested Asaia spp. bacteria, while in the presence of black currant juice we noted the antiadhesive effect for A. bogorensis strains in particular.
Figure 8.
Adhesion of the Asaia spp. strains to PET carrier in M2 medium with sucrose (white bars) supplemented by bilberry (grey bars) and black currant (black bars) juices, evaluated by plate count method (a) and luminometry (b).
The use of fruit juice not only brings antiadhesive effects, but also has other health benefits. The prohealth action of berry juices has been known in folk medicine. However, antiadhesive properties of fruit juices were documented scientifically mainly for cranberry (Vaccinium macrocarpon) [17, 41, 54]. The effect of blueberry constituents on the adhesion of Staph. mutans was also documented [60]. The recent studies are related to the effect of cranberry juice on the growth and adhesion abilities of bacteria Asaia spp. It was documented that, in the presence of cranberry juice, the attachment of A. bogorensis cells to plastic surfaces was significantly lower [19]. However, the mechanisms by which cranberry extracts are effective as antiadhesive agent have not been fully established yet. It is believed that there are two main compounds involved in the inhibition of bacterial attachment: fructose blocking bacterial type 1 fimbriae and proanthocyanidins which bind with type P fimbriae, preventing cells adhesion [41, 61]. The chromatographic analysis of the polyphenols in V. macrocarpon confirmed the presence of type A proanthocyanidin [19, 62]. Thus, we can assume that type A proanthocyanidins present in berries may show an antiadhesive effect to Asaia spp. cells.
4. Conclusions
The results presented in this study suggest that bilberry and black currant juices show high antiadhesive and antibacterial activity against food-spoiled bacteria belonging to the genus Asaia. Particularly V. myrtillus juice characterized by a higher content of polyphenols including A type proanthocyanidin showed strong antiadhesive and bacteriostatic properties. The high content of bioactive compounds with proven health-promoting properties makes them a valuable supplement of soft drinks, as well as interesting alternative to artificial additives to keep the microbial stability of final products.
Acknowledgments
This work was financially supported by the National Science Centre, Poland, Project no. 2015/17/N/NZ9/03635.
Competing Interests
The authors declare that there are no competing interests regarding the publication of this paper.
References
- 1.Seeram N. P. Berry fruits for cancer prevention: current status and future prospects. Journal of Agricultural and Food Chemistry. 2008;56(3):630–635. doi: 10.1021/jf072504n. [DOI] [PubMed] [Google Scholar]
- 2.Szajdek A., Borowska E. J. Bioactive compounds and health-promoting properties of berry fruits: a review. Plant Foods for Human Nutrition. 2008;63(4):147–156. doi: 10.1007/s11130-008-0097-5. [DOI] [PubMed] [Google Scholar]
- 3.Subash S., Essa M. M., Al-Adawi S., Memon M. A., Manivasagam T., Akbar M. Neuroprotective effects of berry fruits on neurodegenerative diseases. Neural Regeneration Research. 2014;9(16):1557–1566. doi: 10.4103/1673-5374.139483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paredes-López O., Cervantes-Ceja M. L., Vigna-Pérez M., Hernández-Pérez T. Berries: improving human health and healthy aging, and promoting quality life—a review. Plant Foods for Human Nutrition. 2010;65(3):299–308. doi: 10.1007/s11130-010-0177-1. [DOI] [PubMed] [Google Scholar]
- 5.Puupponen-Pimiä R., Nohynek L., Meier C., et al. Antimicrobial properties of phenolic compounds from berries. Journal of Applied Microbiology. 2001;90(4):494–507. doi: 10.1046/j.1365-2672.2001.01271.x. [DOI] [PubMed] [Google Scholar]
- 6.Cisowska A., Wojnicz D., Hendrich A. B. Anthocyanins as antimicrobial agents of natural plant origin. Natural Product Communications. 2011;6(1):149–156. [PubMed] [Google Scholar]
- 7.Shmuely H., Burger O., Neeman I., et al. Susceptibility of Helicobacter pylori isolates to the antiadhesion activity of a high-molecular-weight constituent of cranberry. Diagnostic Microbiology and Infectious Disease. 2004;50(4):231–235. doi: 10.1016/j.diagmicrobio.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 8.Vattem D. A., Lin Y.-T., Labbe R. G., Shetty K. Antimicrobial activity against select food-borne pathogens by phenolic antioxidants enriched in cranberry pomace by solid-state bioprocessing using the food grade fungus Rhizopus oligosporus . Process Biochemistry. 2004;39(12):1939–1946. doi: 10.1016/j.procbio.2003.09.032. [DOI] [Google Scholar]
- 9.Caillet S., Côté J., Sylvain J.-F., Lacroix M. Antimicrobial effects of fractions from cranberry products on the growth of seven pathogenic bacteria. Food Control. 2012;23(2):419–428. doi: 10.1016/j.foodcont.2011.08.010. [DOI] [Google Scholar]
- 10.Rafsanjany N., Senker J., Brandt S., Dobrindt U., Hensel A. In vivo consumption of cranberry exerts ex vivo antiadhesive activity against FimH-dominated uropathogenic Escherichia coli: a combined in vivo, ex vivo, and in vitro study of an extract from Vaccinium macrocarpon . Journal of Agricultural and Food Chemistry. 2015;63(40):8804–8818. doi: 10.1021/acs.jafc.5b03030. [DOI] [PubMed] [Google Scholar]
- 11.Qian L., Thomas J., Taylor J. Antimicrobial effects of cranberry juice against common Gram-negative uropathogens in vitro . American Journal of Clinical Pathology. 2014;142, article A074 doi: 10.1093/ajcp/142.suppl1.074. [DOI] [Google Scholar]
- 12.Mathison B. D., Kimble L. L., Kaspar K. L., Khoo C., Chew B. P. Consumption of cranberry beverage improved endogenous antioxidant status and protected against bacteria adhesion in healthy humans: a randomized controlled trial. Nutrition Research. 2014;34(5):420–427. doi: 10.1016/j.nutres.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Kregiel D., Rygała A., Libudzisz Z., Walczak P., Ołtuszak-Walczak E. Asaia lannensis—the spoilage acetic acid bacteria isolated from strawberry-flavored bottled water in Poland. Food Control. 2012;26(1):147–150. doi: 10.1016/j.foodcont.2012.01.020. [DOI] [Google Scholar]
- 14.Moore J. E., McCalmont M., Xu J., Millar B. C., Heaney N. Asaia sp., an unusual spoilage organism of fruit-flavored bottled water. Applied and Environmental Microbiology. 2002;68(8):4130–4131. doi: 10.1128/AEM.68.8.4130-4131.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horsáková I., Voldřich M., Čeřovský M., Sedláčková P., Šicnerová P., Ulbrich P. Asaia sp. as a bacterium decaying the packaged still fruit beverages. Czech Journal of Food Sciences. 2009;27:S362–S365. [Google Scholar]
- 16.Kregiel D. Attachment of Asaia lannensis to materials commonly used in beverage industry. Food Control. 2013;32(2):537–542. doi: 10.1016/j.foodcont.2013.01.037. [DOI] [Google Scholar]
- 17.Kregiel D., Otlewska A., Antolak H. Attachment of Asaia bogorensis originating in fruit-flavored water to packaging materials. BioMed Research International. 2014;2014:6. doi: 10.1155/2014/514190.514190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gutarowska B., Czyżowska A. The ability of filamentous fungi to produce acids on indoor building materials. Annals of Microbiology. 2009;59(4):807–813. doi: 10.1007/bf03179227. [DOI] [Google Scholar]
- 19.Antolak H., Kręgiel D., Czyżowska A. Adhesion of Asaia bogorensis to glass and polystyrene in the presence of cranberry juice. Journal of Food Protection. 2015;78(6):1186–1190. doi: 10.4315/0362-028x.jfp-14-440. [DOI] [PubMed] [Google Scholar]
- 20.Lange J., Wyser Y. Recent innovations in barrier technologies for plastic packaging—a review. Packaging Technology and Science. 2003;16(4):149–158. doi: 10.1002/pts.621. [DOI] [Google Scholar]
- 21.Hori K., Matsumoto S. Bacterial adhesion: from mechanism to control. Biochemical Engineering Journal. 2010;48(3):424–434. doi: 10.1016/j.bej.2009.11.014. [DOI] [Google Scholar]
- 22.Møretrø T., Langsrud S. Listeria monocytogenes: biofilm formation and persistence in food-processing environments. Biofilms. 2004;1(2):107–121. doi: 10.1017/s1479050504001322. [DOI] [Google Scholar]
- 23.Targoński Z., Stój A. Zafałszowania żywności i metody ich wykrywania. Żywność: Nauka, Technologia, Jakość. 2005;4:30–40. [Google Scholar]
- 24.McDougall G. J., Gordon S., Brennan R., Stewart D. Anthocyanin-flavanol condensation products from black currant (Ribes nigrum L.) Journal of Agricultural and Food Chemistry. 2005;53(20):7878–7885. doi: 10.1021/jf0512095. [DOI] [PubMed] [Google Scholar]
- 25.Anttonen M. J., Karjalainen R. O. High-performance liquid chromatography analysis of black currant (Ribes nigrum L.) Fruit phenolics grown either conventionally or organically. Journal of Agricultural and Food Chemistry. 2006;54(20):7530–7538. doi: 10.1021/jf0615350. [DOI] [PubMed] [Google Scholar]
- 26.Gavrilova V., Kajdžanoska M., Gjamovski V., Stefova M. Separation, characterization and quantification of phenolic compounds in blueberries and red and black currants by HPLC-DAD-ESI-MSn. Journal of Agricultural and Food Chemistry. 2011;59(8):4009–4018. doi: 10.1021/jf104565y. [DOI] [PubMed] [Google Scholar]
- 27.Määttä-Riihinen K. R., Kähkönen M. P., Törrönen A. R., Heinonen I. M. Catechins and procyanidins in berries of Vaccinium species and their antioxidant activity. Journal of Agricultural and Food Chemistry. 2005;53(22):8485–8491. doi: 10.1021/jf050408l. [DOI] [PubMed] [Google Scholar]
- 28.Lätti A. K., Riihinen K. R., Kainulainen P. S. Analysis of anthocyanin variation in wild populations of bilberry (Vaccinium myrtillus L.) in Finland. Journal of Agricultural and Food Chemistry. 2008;56(1):190–196. doi: 10.1021/jf072857m. [DOI] [PubMed] [Google Scholar]
- 29.Može Š., Polak T., Gašperlin L., et al. Phenolics in slovenian bilberries (Vaccinium myrtillus L.) and blueberries (Vaccinium corymbosum L.) Journal of Agricultural and Food Chemistry. 2011;59(13):6998–7004. doi: 10.1021/jf200765n. [DOI] [PubMed] [Google Scholar]
- 30.Müller D., Schantz M., Richling E. High performance liquid chromatography analysis of anthocyanins in bilberries (Vaccinium myrtillus L.), blueberries (Vaccinium corymbosum L.), and corresponding juices. Journal of Food Science. 2012;77(4):C340–C345. doi: 10.1111/j.1750-3841.2011.02605.x. [DOI] [PubMed] [Google Scholar]
- 31.Mikkelsen B. B., Poll L. Decomposition and transformation of aroma compounds and anthocyanins during black currant (Ribes nigrum L.) juice processing. Journal of Food Science. 2002;67(9):3447–3455. doi: 10.1111/j.1365-2621.2002.tb09604.x. [DOI] [Google Scholar]
- 32.Wallace T. C. Anthocyanins in cardiovascular disease. Advances in Nutrition. 2011;2(1):1–7. doi: 10.3945/an.110.000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Pascual-Teresa S. Molecular mechanisms involved in the cardiovascular and neuroprotective effects of anthocyanins. Archives of Biochemistry and Biophysics. 2014;559:68–74. doi: 10.1016/j.abb.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 34.Aqil F., Gupta A., Munagala R., et al. Antioxidant and antiproliferative activities of anthocyanin/ellagitannin-enriched extracts from Syzygium cumini L. (Jamun, the Indian Blackberry) Nutrition and Cancer. 2012;64(3):428–438. doi: 10.1080/01635581.2012.657766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsai T.-C., Huang H.-P., Chang Y.-C., Wang C.-J. An anthocyanin-rich extract from Hibiscus sabdariffa linnaeus inhibits N-nitrosomethylurea-induced leukemia in rats. Journal of Agricultural and Food Chemistry. 2014;62(7):1572–1580. doi: 10.1021/jf405235j. [DOI] [PubMed] [Google Scholar]
- 36.Edirisinghe I., Banaszewski K., Cappozzo J., et al. Strawberry anthocyanin and its association with postprandial inflammation and insulin. British Journal of Nutrition. 2011;106(6):913–922. doi: 10.1017/S0007114511001176. [DOI] [PubMed] [Google Scholar]
- 37.Sohn D. W., Bae W. J., Kim H. S., Kim S. W., Kim S. W. The anti-inflammatory and antifibrosis effects of anthocyanin extracted from black soybean on a peyronie disease rat model. Urology. 2014;84(5):1112–1116. doi: 10.1016/j.urology.2014.06.026. [DOI] [PubMed] [Google Scholar]
- 38.Youdim K. A., Shukitt-Hale B., Joseph J. A. Flavonoids and the brain: interactions at the blood-brain barrier and their physiological effects on the central nervous system. Free Radical Biology and Medicine. 2004;37(11):1683–1693. doi: 10.1016/j.freeradbiomed.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 39.Wang D., Xia M., Yan X., et al. Gut microbiota metabolism of anthocyanin promotes reverse cholesterol transport in mice via repressing miRNA-10b. Circulation Research. 2012;111(8):967–981. doi: 10.1161/CIRCRESAHA.112.266502. [DOI] [PubMed] [Google Scholar]
- 40.Lee Y.-L., Cesario T., Wang Y., Shanbrom E., Thrupp L. Antibacterial activity of vegetables and juices. Nutrition. 2003;19(11-12):994–996. doi: 10.1016/j.nut.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 41.Howell A. B. Bioactive compounds in cranberries and their role in prevention of urinary tract infections. Molecular Nutrition and Food Research. 2007;51(6):732–737. doi: 10.1002/mnfr.200700038. [DOI] [PubMed] [Google Scholar]
- 42.Blumberg J. B., Camesano T. A., Cassidy A., et al. Cranberries and their bioactive constituents in human health. Advances in Nutrition. 2013;4(6):618–632. doi: 10.3945/an.113.004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y., Singh A. P., Hurst W. J., Glinski J. A., Koo H., Vorsa N. Influence of degree-of-polymerization and linkage on the quantification of proanthocyanidins using 4-dimethylaminocinnamaldehyde (DMAC) assay. Journal of Agricultural and Food Chemistry. 2016;64(11):2190–2199. doi: 10.1021/acs.jafc.5b05408. [DOI] [PubMed] [Google Scholar]
- 44.Foo L. Y., Lu Y., Howell A. B., Vorsa N. A-type proanthocyanidin trimers from cranberry that inhibit adherence of uropathogenic P-fimbriated Escherichia coli . Journal of Natural Products. 2000;63(9):1225–1228. doi: 10.1021/np000128u. [DOI] [PubMed] [Google Scholar]
- 45.Schmidt B. M., Howell A. B., McEniry B., et al. Effective separation of potent antiproliferation and antiadhesion components from wild blueberry (Vaccinium angustifolium Ait.) fruits. Journal of Agricultural and Food Chemistry. 2004;52(21):6433–6442. doi: 10.1021/jf049238n. [DOI] [PubMed] [Google Scholar]
- 46.Cuevas-Rodríguez E. O., Yousef G. G., García-Saucedo P. A., López-Medina J., Paredes-López O., Lila M. A. Characterization of anthocyanins and proanthocyanidins in wild and domesticated mexican blackberries (Rubus spp.) Journal of Agricultural and Food Chemistry. 2010;58(12):7458–7464. doi: 10.1021/jf101485r. [DOI] [PubMed] [Google Scholar]
- 47.Gu L. W., Kelm M. A., Hammerstone J. F., et al. Screening of foods containing proanthocyanidins and their structural characterization using LC-MS/MS and thiolytic degradation. Journal of Agricultural and Food Chemistry. 2003;51(25):7513–7521. doi: 10.1021/jf034815d. [DOI] [PubMed] [Google Scholar]
- 48.Määttä-Riihinen K. R., Kamal-Eldin A., Mattila P. H., González-Paramás A. M., Törrönen R. Distribution and contents of phenolic compounds in eighteen scandinavian berry species. Journal of Agricultural and Food Chemistry. 2004;52(14):4477–4486. doi: 10.1021/jf049595y. [DOI] [PubMed] [Google Scholar]
- 49.Wu X., Gu L., Prior R. L., McKay S. Characterization of anthocyanins and proanthocyanidins in some cultivars of Ribes, Aronia, and Sambucus and their antioxidant capacity. Journal of Agricultural and Food Chemistry. 2004;52(26):7846–7856. doi: 10.1021/jf0486850. [DOI] [PubMed] [Google Scholar]
- 50.Buendía B., Gil M. I., Tudela J. A., et al. HPLC-MS analysis of proanthocyanidin oligomers and other phenolics in 15 strawberry cultivars. Journal of Agricultural and Food Chemistry. 2010;58(7):3916–3926. doi: 10.1021/jf9030597. [DOI] [PubMed] [Google Scholar]
- 51.White B. L., Howard L. R., Prior R. L. Release of bound procyanidins from cranberry pomace by alkaline hydrolysis. Journal of Agricultural and Food Chemistry. 2010;58(13):7572–7579. doi: 10.1021/jf100700p. [DOI] [PubMed] [Google Scholar]
- 52.Howard L. R., Castrodale C., Brownmiller C., Mauromoustakos A. Jam processing and storage effects on blueberry polyphenolics and antioxidant capacity. Journal of Agricultural and Food Chemistry. 2010;58(7):4022–4029. doi: 10.1021/jf902850h. [DOI] [PubMed] [Google Scholar]
- 53.Madhavi D. L., Bomser J., Smith M. A. L., Singletary K. Isolation of bioactive constituents from Vaccinium myrtillus (bilberry) fruits and cell cultures. Plant Science. 1998;131(1):95–103. doi: 10.1016/s0168-9452(97)00241-0. [DOI] [Google Scholar]
- 54.Burger O., Weiss E., Sharon N., Tabak M., Neeman I., Ofek I. Inhibition of Helicobacter pylori adhesion to human gastric mucus by a high-molecular-weight constituent of cranberry juice. Critical Reviews in Food Science and Nutrition. 2002;42(3):279–284. doi: 10.1080/10408390209351916. [DOI] [PubMed] [Google Scholar]
- 55.Scalbert A. Antimicrobial properties of tannins. Phytochemistry. 1991;30(12):3875–3883. doi: 10.1016/0031-9422(91)83426-L. [DOI] [Google Scholar]
- 56.Nohynek L. J., Alakomi H.-L., Kähkönen M. P., et al. Berry phenolics: antimicrobial properties and mechanisms of action against severe human pathogens. Nutrition and Cancer. 2006;54(1):18–32. doi: 10.1207/s15327914nc5401_4. [DOI] [PubMed] [Google Scholar]
- 57.Helander I. M., Alakomi H.-L., Latva-Kala K., et al. Characterization of the action of selected essential oil components on gram-negative bacteria. Journal of Agricultural and Food Chemistry. 1998;46(9):3590–3595. doi: 10.1021/jf980154m. [DOI] [Google Scholar]
- 58.Puupponen-Pimiä R., Nohynek L., Alakomi H.-L., Oksman-Caldentey K.-M. Bioactive berry compounds—novel tools against human pathogens. Applied Microbiology and Biotechnology. 2005;67(1):8–18. doi: 10.1007/s00253-004-1817-x. [DOI] [PubMed] [Google Scholar]
- 59.He Q., Lv Y., Yao K. Effects of tea polyphenols on the activities of α-amylase, pepsin, trypsin and lipase. Food Chemistry. 2006;101(3):1178–1182. doi: 10.1016/j.foodchem.2006.03.020. [DOI] [Google Scholar]
- 60.Weiss E. I., Lev-Dor R., Sharon N., Ofek I. Inhibitory effect of a high-molecular-weight constituent of cranberry on adhesion of oral bacteria. Critical Reviews in Food Science and Nutrition. 2002;42(3):285–292. doi: 10.1080/10408390209351917. [DOI] [PubMed] [Google Scholar]
- 61.Nicolosi D., Tempera G., Genovese C., Furneri P. M. Anti-adhesion activity of A2-type proanthocyanidins (a cranberry major component) on uropathogenic E. coli and P. mirabilis strains. Antibiotics. 2014;3(2):143–154. doi: 10.3390/antibiotics3020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Krueger C. G., Reed J. D., Feliciano R. P., Howell A. B. Quantifying and characterizing proanthocyanidins in cranberries in relation to urinary tract health. Analytical and Bioanalytical Chemistry. 2013;405(13):4385–4395. doi: 10.1007/s00216-013-6750-3. [DOI] [PubMed] [Google Scholar]