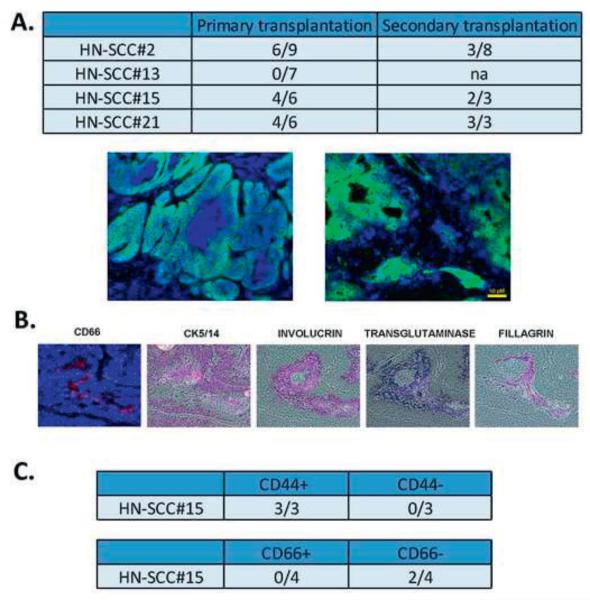
FIGURE 5. Tumorigenicity of CSC in HNSCC.
5A. 106 tumor cells were injected subcutaneously into the neck region of immunodeficient mice. Tumors were harvested and stained by immunohistochemistry. HLA-ABC (green) staining specifically identified the human tumor cells in the mouse. The Figure represents two distinct tumors, each showing different architecture, similar to the original tumors.
5B. IHC showing reconstitution of the original heterogeneity in HN-SCC#2. The xenograft was stained with antibodies against CD66, CK5/14, involucrin, transglutaminase and filagrin. Histopathologic analysis of the xenografts demonstrated moderately differentiated squamous cell carcinoma-like tissue morphology without evidence of invasion.
5C. CD44 and CD66 subpopulations were sorted and injected into immunodeficient mice. CD44 sorted cells show tumorigenicity when compared to CD66 cells.
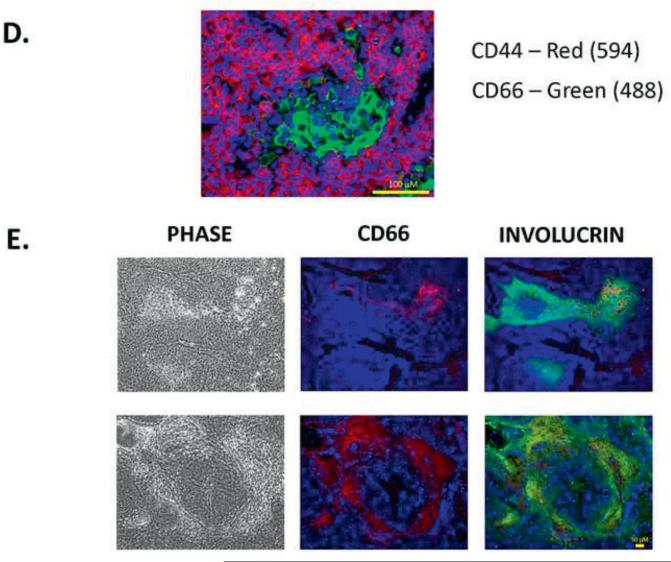
5D. Double immunostaining of xenografts derived from HN-SCC#2 (CD44, CD66). The staining shows distinct areas of CD44+ and CD66+, suggesting that CSC and differentiating cells are mutually exclusive.
5E. Double immunostaining of xenografts, derived from HN-SCC#2 (CD66, involucrin). The staining shows co-localization of CD66+ and involucrin+ in the center of the squamous tumor islands, confirming that CD66 is a differentiation marker in HNSCC.
5F. Flow cytometric analyses of CD44 and CD66 staining in four early passage HNSCC cultures. The results suggest that our culture approach selects for a CD44+/CD66− subpopulation of cells.
5G. CD44 subpopulations or CD66 subpopulations were sorted, plated on 96-well plates, and assessed for CSC frequency by limiting dilution analysis. The results show higher colony forming units (CFU) for both the CD44+ and CD66− sorted populations compared to the CD44− and CD66+ sorted populations.