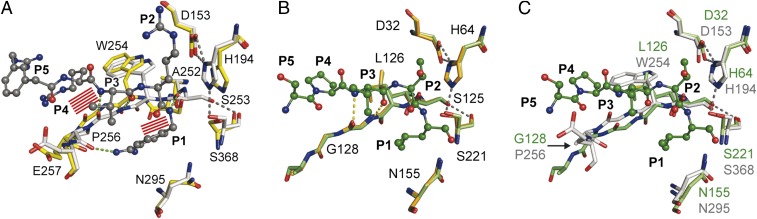
Fig. 2.
Structural comparison of substrates binding to the active sites of furin and to subtilisin Carlsberg. (A) Structural alignment of selected residues of unliganded furin (yellow carbons) and inhibitor-bound furin (gray carbons; inhibitor: ball-and-stick). Steric clashes between bound inhibitor/substrate and unliganded furin are highlighted as red line patterns. (B) Structural alignment of unliganded subtilisin (orange carbons) and inhibitor-bound subtilisin (green carbons). (C) Structural alignment of inhibitor-bound subtilisin (green carbons) and furin (gray-colored stick model). Important interactions are always highlighted by dashes.