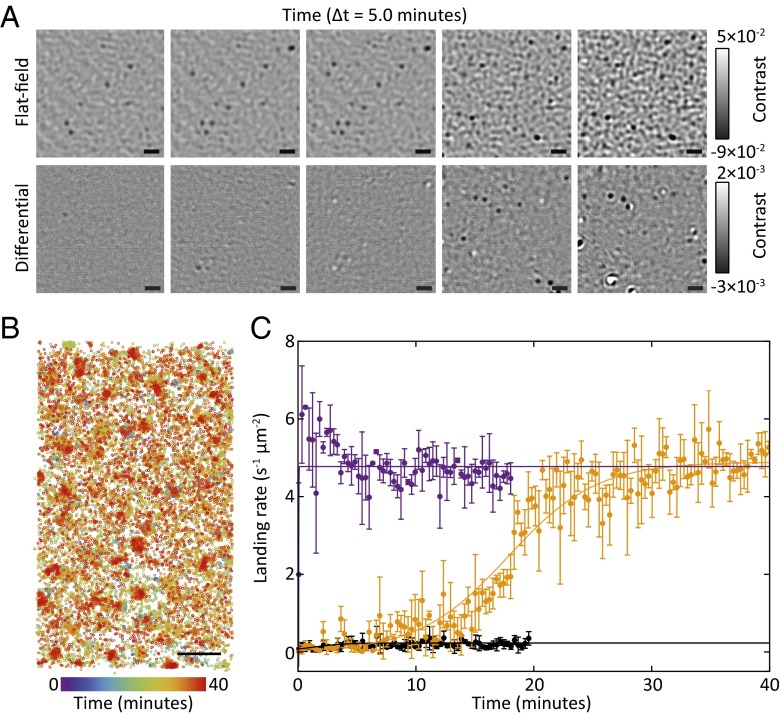
Fig. 2.
Quantification of reaction kinetics by label-free superresolution imaging. The binding of aggregates of 3 to a glass surface is monitored by iSCAT. The binding and unbinding of particles is detected as a change in the local refractive index and counted, allowing quantification of the binding/unbinding rates per unit area. (A, Top) Flat-field images of aggregates of 3 binding to a microscope cover glass over 25 min. (A, Bottom) Corresponding background-subtracted images, highlighting binding (dark colors) and unbinding (white) events of single aggregates to the surface. Images are taken from the same dataset as the orange line in C. Each image is the average of 150 frames. (B) Superresolution map identifying the center of mass for each binding event over time. Counting each binding event in this map per unit time gives the data shown in C. Data are the same as the orange line in C albeit cropped to a 8.1 × 4.8 μm window. (Scale bars: 1 μm.) Kinetic curves for these reactions and additional replicates are included in Figs. S8–S10. (C) Characterization of reaction kinetics by counting the number of binding events per unit time and area. Background-subtracted images (illustrated in A, Bottom) are analyzed and each binding/unbinding event is counted to give the kinetic curves shown. Data points with error bars represent the average and SD of three consecutive 1-s measurements sampled once every 6 s. Solid lines are fits to sigmoidal kinetics for the reaction between 1 and 2 (orange) and the reaction between 1 and 2 seeded with 3 (purple). The seeded reaction features a high rate of reaction immediately upon addition of 1, without the lag period required to build up product/catalyst as observed in unseeded reactions. The black line and corresponding data points refer to the negative control, consisting of thiol 1 and an aqueous solution of Cs2CO3. Note that solid lines do not represent a detailed kinetic model and are intended only to highlight major trends.