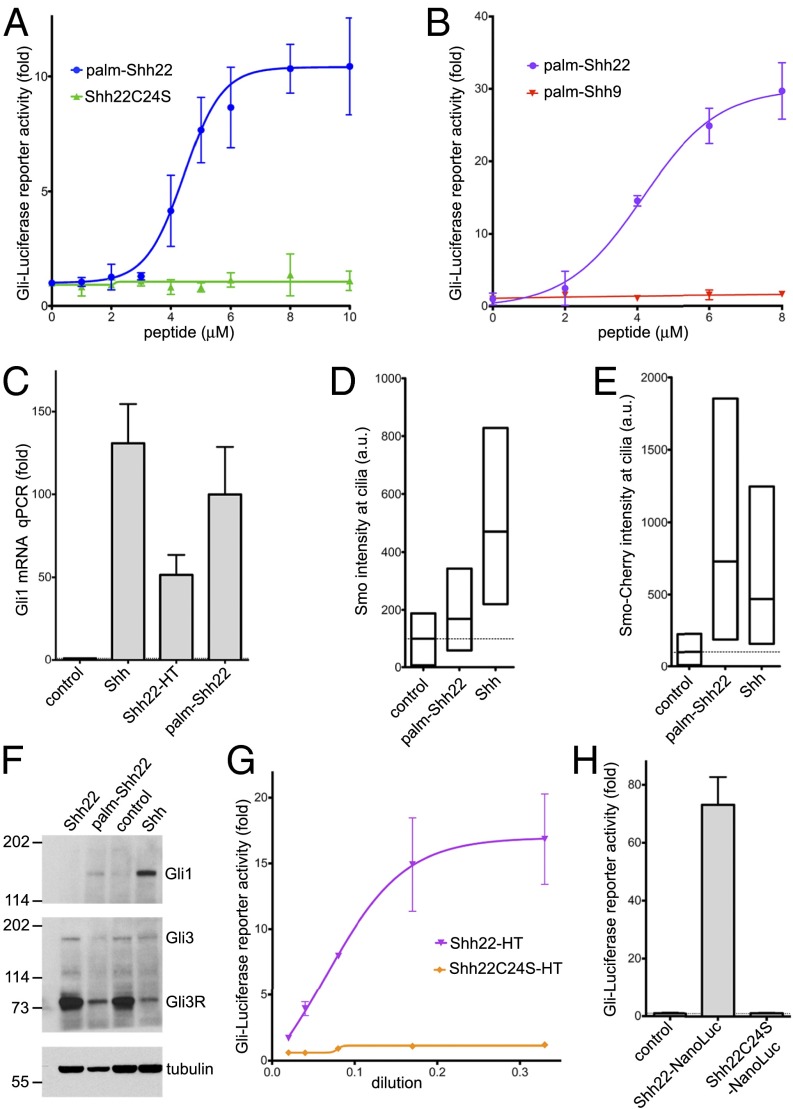
Fig. 1.
A palmitoylated Shh peptide activates Hh signaling. (A) Shh Light II cells were treated with various concentrations of the synthetic peptides palm-Shh22 or Shh22C24S, and Hh pathway activity was measured by luciferase assay. Error bars represent SD (n = 3). Palm-Shh22 activates Hh signaling, whereas nonpalmitoylated Shh22C24S is inactive. (B) As in A, but with treatment with palm-Shh22 or palm-Shh9. Palm-Shh9 does not activate Hh signaling, in contrast to palm-Shh22. (C) NIH 3T3 cells were incubated with control media, Shh ligand, Shh22-HT, or palm-Shh22 (5 μM). Gli1 transcripts were measured by quantitative RT-PCR. Error bars represent SD (n = 3). Shh22-HT, palm-Shh22, and Shh stimulate Gli1 transcription. (D) NIH 3T3 cells were incubated with control media, palm-Shh22 (1 μM), or Shh. Endogenous Smo localization in primary cilia was measured by immunofluorescence and automated image analysis. The graph shows box plots of Smo fluorescence intensity in cilia, indicating the median and the 25th and 75th percentile of the distribution (n > 300 cilia). Palm-Shh22 recruits Smo to cilia, although to a lesser extent than Shh. (E) As in D, but with cells stably expressing mCherry-tagged Smo. (F) As in D, but cells were analyzed by immunoblotting with anti-Gli1 and anti-Gli3 antibodies. Blotting for tubulin served as loading control. Both palm-Shh22 and Shh reduce Gli3R levels and induce Gli1 protein accumulation, while the nonpalmitoylated peptide, Shh22, is inactive. (G) As in A, but cells were treated with various concentrations of Shh22-HT, or the palmitoylation site mutant, Shh22C24S-HT. Shh22-HT activates Hh signaling in a palmitate-dependent manner. (H) As in A, but with incubation with control media, or the secreted fusions Shh22-NanoLuc and Shh22C24S-NanoLuc. Shh22-NanoLuc activates Hh signaling in a palmitate-dependent manner.